Effect of pH on the Structure, Functional Properties and Rheological Properties of Collagen from Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Skin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield
2.2. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS–PAGE) of GHSC
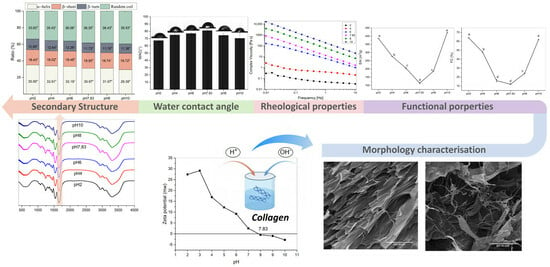
2.3. Zeta Potential
2.4. Morphology Characterisation
2.5. Secondary Structure
2.6. Water Contact Angle (WCA)
2.7. Functional Properties
2.7.1. Emulsifying Activity Index (EAI) and Emulsifying Stability Index (ESI)
2.7.2. Foaming Capacity (FC) and Foaming Stability (FS)
2.8. Rheological Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of GHSC
3.3. Yield of GHSC
3.4. SDS–PAGE
3.5. Zeta Potential
3.6. Morphological Characteristics
3.7. FTIR Spectroscopy
3.8. WCA
3.9. Functional Properties
3.9.1. EAI and ESI
3.9.2. FC and FS
3.10. Rheological Properties
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sarker, P.; Jani, P.K.; Hsiao, L.C.; Rojas, O.J.; Khan, S.A. Interacting collagen and tannic acid particles: Uncovering pH-dependent rheological and thermodynamic behaviors. J. Colloid Interface Sci. 2023, 650, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.C.; Islam, M.R.; Sadia, S.; Yeasmin, M.; Park, J.S.; Lee, H.J.; Chun, B.S. Trash to treasure: An up-to-date understanding of the valorization of seafood by-products, targeting the major bioactive compounds. Mar. Drugs 2023, 21, 485. [Google Scholar] [CrossRef]
- Yao, L.Y.; Hu, Y.; Liu, Z.; Ding, X.; Tian, J.; Xiao, J.X. Luminescent lanthanide–collagen peptide framework for pH-controlled drug delivery. Mol. Pharm. 2019, 6, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Wang, G.Y.; Li, Y.S. Preparation and characterization of thermally stable collagens from the scales of lizardfish (Synodus macrops). Mar. Drugs 2021, 19, 597. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.Z.; Zhang, W.H.; Shi, B. Effect of pH on structure and stability of collagen-like peptide: Insight from molecular dynamics simulation. J. Theor. Comput. Chem. 2011, 10, 245–259. [Google Scholar] [CrossRef]
- Lai, G.L.; Li, Y.; Li, G.Y. Effect of concentration and temperature on the rheological behavior of collagen solution. Int. J. Biol. Macromol. 2008, 42, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Li, L.; Yi, R.Z.; Xu, N.H.; Gao, R.; Hong, B.H. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Mo, C.; Wang, Q.L.; Li, G.F.; Dong, W.W.; Liang, F.; Wu, C.X.; Wang, Z.P.; Wang, Y.F. Extraction and characterization of pepsin- and acid-soluble collagen from the swim bladders of Megalonibea fusca. Mar. Drugs 2023, 21, 159. [Google Scholar] [CrossRef]
- Gao, X.Y.; He, J.L.; Chen, J.D.; Zheng, Y.; Li, Y.S.; Ye, T. Double-spotted pufferfish (Takifugu bimaculatus) skin collagen: Preparation, structure, cytocompatibility, rheological, and functional properties. Arab. J. Chem. 2023, 16, 104402. [Google Scholar] [CrossRef]
- Ran, X.G.; Wang, L.Y. Use of ultrasonic and pepsin treatment in tandem for collagen extraction from meat industry by-products. J. Sci. Food Agric. 2014, 94, 585–590. [Google Scholar] [CrossRef]
- Tien, W.B.; Chen, M.T.; Yao, P.C. Effects of pH and temperature on microstructure and morphology of hydroxyapatite/collagen composites synthesized in vitro. Mater. Sci. Eng. C 2012, 32, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.R.; Duarte, L.P.; Schmidt, M.M.; Cansian, R.L.; Fernandes, I.A.; Mello, R.D.; Demiate, I.M.; Dornelles, R.C.P. Extraction and characterization of collagen from sheep slaughter by-products. Waste Manag. 2022, 102, 838–846. [Google Scholar] [CrossRef]
- Chen, J.D.; Li, J.Y.; Li, Z.B.; Yi, R.Z.; Shi, S.J.; Wu, K.Y.; Li, Y.S.; Wu, S.J. Physicochemical and functional properties of type I collagens in red stingray (Dasyatis akajei) skin. Mar. Drugs 2019, 17, 558. [Google Scholar] [CrossRef]
- Wang, Y.L.; Song, L.; Guo, C.C.; Ji, R.M.T. Identification and characterization of collagen from bactrian camel (Camelus bactrianus) hoof. Foods 2023, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Toniasso, D.P.W.; Giacomelli da Silva, G.; De Souza Brum, B., Jr.; Sabrina, S.; Emanuelli, T.; Kubota, E.H.; Dornelles, R.C.P.; Mello, R. Collagen extracted from rabbit: Meat and by-products: Isolation and physicochemical assessment. Food Res. Int. 2022, 162, 111967. [Google Scholar] [CrossRef]
- Devita, L.; Nurilmala, M.; Lioe, H.N.; Suhartono, M.T. Chemical and antioxidant characteristics of skin-derived collagen obtained by acid-enzymatic hydrolysis of bigeye tuna (Thunnus obesus). Mar. Drugs 2021, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.G.; Wang, Y.; Xu, W.B.; Qin, B.; Ying, N.; Song, X.F.; Yue, Y.F.; Wang, X.S.; Zhang, B.B.; Wu, Y.Q. Transcriptome analysis of greenfin horse-faced filefish (Thamnaconus septentrionalis) gills in response to Amyloodinium ocellatum (AO) Infection. Fishes 2022, 7, 252. [Google Scholar] [CrossRef]
- Bian, L.; Li, F.H.; Ge, J.L.; Wang, P.F.; Chang, Q.; Zhang, S.N.; Li, J.; Liu, C.L.; Liu, K.; Liu, X.T.; et al. Chromosome-level genome assembly of the greenfin horse-faced filefish (Thamnaconus septentrionalis) using Oxford Nanopore PromethION sequencing and Hi-C technology. Mol. Ecol. Resour. 2020, 20, 1069–1079. [Google Scholar] [CrossRef]
- Wang, M.C.; Li, J.; Li, W.J.; Du, Z.N.; Qin, S. Preparation and characterization of novel poly (vinyl alcohol)/collagen double-network hydrogels. Int. J. Biol. Macromol. 2018, 118, 41–48. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.Q.; Lin, M.G.; Zhao, R.Y.; Tang, C.H.; Luo, L.; Liu, L. Complex coacervation of chitosan and soy globulins in aqueous solution: A electrophoretic mobility and light scattering study. Int. J. Food Sci. Technol. 2011, 46, 1363–1369. [Google Scholar] [CrossRef]
- Wang, X.C.; Wang, W.N.; Liu, X.H.; Wang, Y.Y. Amphoteric functional polymers for leather wet finishing auxiliaries: A review. Polym. Adv. Technol. 2021, 32, 1951–1964. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Z.; Ma, Y.; Chiou, B.S.; Liu, F.; Zhong, F. Modulating physicochemical properties of collagen films by cross-linking with glutaraldehyde at varied pH values. Food Hydrocoll. 2022, 124, 107270. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Chun, B.S. Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus). Int. J. Biol. Macromol. 2019, 135, 668–676. [Google Scholar] [CrossRef]
- Chen, J.D.; Li, M.; Yi, R.Z.; Bai, K.K.; Wang, G.G.; Tan, R.; Sun, S.S.; Xu, N.H. Electrodialysis extraction of pufferfish skin (Takifugu flavidus): A promising source of collagen. Mar. Drugs 2019, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Bielska, P.; Cais-Sokolinska, D.; Dwiecki, K. Effects of heat treatment duration on the electrical properties, texture and color of polymerized whey protein. Molecules 2022, 27, 6395. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Chang, S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Mu, J.L.; Qi, Y.W.; Gong, K.X.; Chen, Z.Z.; Brennan, M.A.; Ma, Q.Y.; Wang, J.; Brennan, C.S. Effects of quinoa flour (Chenopodium quinoa Willd) substitution on wheat flour characteristics. Curr. Res. Food Sci. 2023, 7, 100556. [Google Scholar] [CrossRef]
- Li, Y.S.; Yang, L.H.; Wu, S.J.; Chen, J.D.; Lin, H.W. Structural, functional, rheological, and biological properties of the swim bladder collagen extracted from grass carp (Ctenopharyngodon idella). LWT Food Sci. Technol. 2022, 153, 112518. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-waterhouse, D.X.; Cui, C.; Wang, W.; Dong, K.M. Modification of soy protein isolate by glutaminase for nanocomplexation with curcumin. Food Chem. 2018, 268, 504–512. [Google Scholar] [CrossRef]
- Zhou, X.F.; Zheng, Y.R.; Zhong, Y.; Wang, D.F.; Deng, Y. Casein-hempseed protein complex via cross-link catalyzed by transglutaminase for improving structural, rheological, emulsifying and gelation properties. Food Chem. 2022, 383, 132366. [Google Scholar] [CrossRef]
- Dhakal, D.; Koomsap, P.; Lamichhane, A.; Sadiq, M.B.; Anal, A.K. Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Biosci. 2018, 23, 23–30. [Google Scholar] [CrossRef]
- Ngui, S.P.; Nyobe, C.E.; Bassogog, C.B.B.; Tang, E.N.; Minka, S.R.; Mune, M.A.M. Influence of pH and temperature on the physicochemical and functional properties of Bambara bean protein isolate. Heliyon 2021, 7, e07824. [Google Scholar] [CrossRef] [PubMed]
- Saatchi, A.; Kiani, H.; Labbafi, M. A new functional protein-polysaccharide conjugate based on protein concentrate from sesame processing by-products: Functional and physico-chemical properties. Int. J. Biol. Macromol. 2019, 122, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Pan, D.D.; Xia, Q.; Cao, J.X.; Zhou, C.Y.; He, J.; Sun, Y.Y.; Xu, S.L. Impact of pH-dependent succinylation on the structural features and emulsifying properties of chicken liver protein. Food Chem. 2021, 358, 129868. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.J.; Zhang, X.X.; Han, X.; Wang, Z.P.; He, R.H.; Ma, H.L. Structure and functional characteristics of rapeseed protein isolate-dextran conjugates. Food Hydrocoll. 2018, 82, 329–337. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, L.P.; Zhuang, Y.L.; Gu, Y.; Cheng, G.G.; Fan, X.J.; Ding, Y.Y.; Liu, H.T. Protein-stabilized emulsion gels with improved emulsifying and gelling properties for the delivery of bioactive ingredients: A review. Foods 2023, 12, 2703. [Google Scholar] [CrossRef] [PubMed]
- Gahruie, H.H.; Niakousari, M.; Parastouei, K.; Mokhtarian, M.; Eş, I.; Khaneghah, A.M. Co-encapsulation of vitamin D3 and saffron petals’ bioactive compounds in nanoemulsions: Effects of emulsifier and homogenizer types. J. Food Process. Preserv. 2020, 44, e14629. [Google Scholar] [CrossRef]
- Mao, X.Y.; Hua, Y.F. Composition, structure and functional properties of protein concentrates and isolates produced from walnut (Juglans regia L.). Int. J. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Liang, Z.Q.; Zhang, C.Y.; Hao, S.Q.; Han, H.Y.; Du, P.; Li, A.L.; Shao, H.; Li, C.; Liu, L.B. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT Food Sci. Technol. 2021, 152, 112272. [Google Scholar] [CrossRef]
- Liao, L.; Liu, T.X.; Zhao, M.M.; Cui, C.; Yuan, B.E.; Tang, S.; Yang, F. Functional, nutritional and conformational changes from deamidation of wheat gluten with succinic acid and citric acid. Food Chem. 2010, 123, 123–130. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Iordanopoulou, A.; Bouropoulos, N.; Meikopoulos, T.; Gika, H.; Ritzoulis, C.; Andreadis, D.; Karavasili, C.; Fatouros, D.G. Pediatric and geriatric-friendly buccal foams: Enhancing omeprazole delivery for patients encountering swallowing difficulties. J. Pharm. Sci. 2023, 112, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Pant, A.; Pojchanun, K.; Lee, C.P.; An, J.; Hashimoto, M.; Tan, U.X.; Leo, C.H.; Wong, G.; Chua, C.K.; et al. Three-dimensional printing of food foams stabilized by hydrocolloids for hydration in dysphagia. Int. J. Bioprinting 2021, 7, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.F.; Li, S.Y.; Tian, M.; Yang, P.; Ding, Y.J.; Wang, Y.W.; Duan, G.M.; Zhang, D.H.; Chen, B.; Tan, Q. Facile preparation of a novel nanoemulsion based hyaluronic acid hydrogel loading with poria cocos triterpenoids extract for wound dressing. Int. J. Biol. Macromol. 2023, 226, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.D.F.; Carvalho, S.M.; Mansur, H.S.; Pereira, M.M. Thermogelling chitosan-collagen-bioactive glass nanoparticle hybrids as potential injectable systems for tissue engineering. Mater. Sci. Eng. C 2016, 58, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.C.; Yang, J.H.; Lan, F.; Zheng, Z.G.; Dai, L.B.; Zhang, M. Insight into the rheological behaviors of a polyanionic collagen fabricated with poly(γ-glutamic acid)-NHS ester. Biotechnol. Appl. Biochem. 2019, 66, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Milan, E.P.; Martins, V.C.A.; Horn, M.M.; Plepis, A.M.G. Influence of blend ratio and mangosteen extract in chitosan/collagen gels and scaffolds: Rheological and release studies. Carbohydr. Polym. 2022, 292, 119647. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zhao, W.H.; Chi, C.F.; Zhao, Y.Q.; Wang, B. Preparation, physicochemical and antioxidant properties of acid- and pepsin-soluble collagens from the swim bladders of miiuy croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 161. [Google Scholar] [CrossRef]
- Wang, J.; Pei, X.L.; Liu, H.Y.; Zhou, D. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus). Int. J. Biol. Macromol. 2018, 106, 544–550. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, Z.H.; Hou, H.; Zhao, X.; Li, B.F.; Zhao, T.F.; Liu, L.Y. Characterization of acid- and pepsin-soluble collagens from the cuticle of Perinereis nuntia (Savigny). Food Biophys. 2018, 13, 274–283. [Google Scholar] [CrossRef]
- Deng, L.L.; Zhang, X.; Li, Y.; Que, F.; Kang, X.F.; Liu, Y.Y.; Feng, F.Q.; Zhang, H. Characterization of gelatin/zein nanofibers by hybrid electrospinning. Food Hydrocoll. 2018, 75, 72–80. [Google Scholar] [CrossRef]
- Ogunwolu, S.O.; Henshaw, F.O.; Mock, H.P.; Santros, A.; Awonorin, S.O. Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut. Food Chem. 2009, 115, 852–858. [Google Scholar] [CrossRef]
- Deng, Y.J.; Huang, L.X.; Zhang, C.H.; Xie, P.J.; Cheng, J.; Wang, X.J.; Li, S.H. Physicochemical and functional properties of chinese quince seed protein isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Li, Y.; Chen, J. Effect of pH on the Structure, Functional Properties and Rheological Properties of Collagen from Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Skin. Mar. Drugs 2024, 22, 45. https://doi.org/10.3390/md22010045
Wu K, Li Y, Chen J. Effect of pH on the Structure, Functional Properties and Rheological Properties of Collagen from Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Skin. Marine Drugs. 2024; 22(1):45. https://doi.org/10.3390/md22010045
Chicago/Turabian StyleWu, Kunyuan, Yushuang Li, and Junde Chen. 2024. "Effect of pH on the Structure, Functional Properties and Rheological Properties of Collagen from Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Skin" Marine Drugs 22, no. 1: 45. https://doi.org/10.3390/md22010045
APA StyleWu, K., Li, Y., & Chen, J. (2024). Effect of pH on the Structure, Functional Properties and Rheological Properties of Collagen from Greenfin Horse-Faced Filefish (Thamnaconus septentrionalis) Skin. Marine Drugs, 22(1), 45. https://doi.org/10.3390/md22010045