Anithiactin D, a Phenylthiazole Natural Product from Mudflat-Derived Streptomyces sp., Suppresses Motility of Cancer Cells
Abstract
:1. Introduction

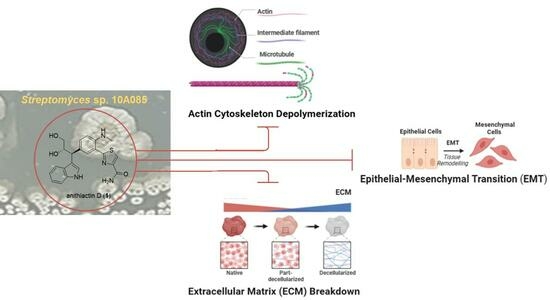
2. Results
Structure Elucidation
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strain Isolation
3.3. Fermentation, Extraction, and Purification
3.4. ECD Calculation
3.5. Cell Culture
3.6. MTT Assay
3.7. Cell Migration and Invasion Assays
3.8. Quantitative Real-Time PCR
3.9. Affinity-Precipitation of Cellular GTPases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.iarc.who.int/featured-news/world-cancer-day-2022/ (accessed on 2 October 2022).
- Weiss, F.; Lauffenburger, D.; Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 2022, 22, 157–173. [Google Scholar] [CrossRef]
- Bill, R.; Christofori, G. The Relevance of EMT in Breast Cancer Metastasis: Correlation or Causality? FEBS Lett. 2015, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kashaninejad, N.; Nguyen, N.-T. RhoA and Rac1 in liver cancer cells: Induction of overexpression using mechanical stimulation. Micromachines 2020, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, I.; Patil, R.S.; Kang, S.; Lee, J.; Choi, H.; Kim, M.-S.; Nam, S.-J.; Kang, H. Anithiactins A–C, modified 2-phenylthiazoles from a mudflat-derived Streptomyces sp. J. Nat. Prod. 2014, 77, 2716–2719. [Google Scholar] [CrossRef]
- Fu, P.; MacMillan, J.B. Thiasporines A–C, thiazine and thiazole derivatives from a marine-derived Actinomycetospora chlora. J. Nat. Prod. 2015, 78, 548–551. [Google Scholar] [CrossRef]
- Seitz, T.; Fu, P.; Haut, F.-L.; Adam, L.; Habicht, M.; Lentz, D.; MacMillan, J.B.; Christmann, M. One-pot synthesis of 5-hydroxy-4H-1,3-thiazin-4-ones: Structure revision, synthesis, and nmr shift dependence of thiasporine A. Org. Lett. 2016, 18, 3070–3073. [Google Scholar] [CrossRef]
- Lamb, R.A.; Badart, M.P.; Swaney, B.E.; Gai, S.; Baird, S.K.; Hawkins, B.C. The synthesis and biological evaluation of anithiactin A/thiasporine C and analogues. Aust. J. Chem. 2015, 68, 1829–1833. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z.; Zhao, X.; Shan, Q.; He, P.; Du, Y.; Wang, Y. Simple and efficient synthesis of anithiactins A–C, thiasporine A and their potent antitumor 2,4-linked oligothiazole derivatives. ChemistrySelect 2019, 4, 742–746. [Google Scholar] [CrossRef]
- Vaaland, I.C.; Lindbäck, E.; Sydnes, M.O. Total synthesis of anithiactins A–C and thiasporine A. Tetrahedron Lett. 2019, 60, 610–612. [Google Scholar] [CrossRef]
- Cox, C.D.; Rinehart, K.L., Jr.; Moore, M.L.; Cook, J.C., Jr. Pyochelin: Novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1981, 78, 4256–4260. [Google Scholar] [CrossRef] [PubMed]
- Kumaria, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Urbina, K.; Tresp, D.; Sipps, K.; Szostak, M. Recent advances in metal-catalyzed functionalization of indoles. Adv. Synth. Catal. 2021, 363, 2723–2727. [Google Scholar] [CrossRef]
- Ozaki, T.; Minami, A.; Oikawa, H. Biosynthesis of indole diterpenes: A reconstitution approach in a heterologous host. Nat. Prod. Rep. 2023, 40, 202–213. [Google Scholar] [CrossRef]
- Hu, Y.; Potts, M.B.; Colosimo, D.; Herrera-Herrera, M.L.; Legako, A.G.; Yousufuddin, M.; White, M.A.; MacMillan, J.B. Discoipyrroles A−D: Isolation, structure determination, and synthesis of potent migration inhibitors from Bacillus hunanensis. J. Am. Chem. Soc. 2013, 135, 13387–13392. [Google Scholar] [CrossRef]
- Kumagai, H.; Iijima, M.; Dobashi, K.; Naganawa, H.; Sawa, T.; Hamada, M.; Ishizuka, M.; Takeuchi, T. Cytoblastin, a low molecular weight immunomodulator produced by Streptoverticillium eurocidicum. J. Antibiot. 1991, 44, 1029–1032. [Google Scholar] [CrossRef]
- Kaplan, A.R.; Musaev, D.G.; Wuest, W.M. Pyochelin biosynthetic metabolites bind iron and promote growth in pseudomonads demonstrating siderophore-like activity. ACS Infect. Dis. 2021, 7, 544–551. [Google Scholar] [CrossRef]
- Mevers, E.; Saurí, J.; Helfrich, E.J.N.; Henke, M.; Barns, K.J.; Bugni, T.S.; Andes, D.; Currie, C.R.; Clardy, J. Pyonitrins A−D: Chimeric natural products produced by Pseudomonas protegens. J. Am. Chem. Soc. 2019, 141, 17098–17101. [Google Scholar] [CrossRef]
- Clark, A.G.; Vignjevic, D.M. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 2015, 36, 13–22. [Google Scholar] [CrossRef]
- Novikov, N.M.; Zolotaryova, S.Y.; Gautreau, A.M.; Denisov, E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer 2020, 124, 102–114. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef]
- Yadav, S.; Barton, M.; Nguyen, N.T. Stretching induces overexpression of RhoA and Rac1 GTPases in breast cancer cells. Adv. Biosyst. 2020, 4, 190–222. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of gelatinases MMP-2 and MMP-9 in healthy and complicated pregnancy and their future potential as preeclampsia biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, M.; Tsao, S.W.; Man, K.; Zhang, Z.; Feng, Y. Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma. PLoS ONE 2012, 7, e46318. [Google Scholar] [CrossRef]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 18 July 2023).
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nguyen, T.T.; Jeong, M.H.; Crişan, F.; Yu, Y.H.; Ha, H.H.; Choi, K.H.; Jeong, H.G.; Jeong, T.C.; Lee, K.Y.; et al. Inhibitory activity of (+)-usnic acid against non-small cell lung cancer cell motility. PLoS ONE 2016, 11, e0146575. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Overall, C.M.; López-Otín, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Yang, I. Novel Secondary Metabolites from Marine Sediment-Derived Actinomycetes. Ph.D. Thesis, Seoul National University, Seoul, Republic of Korea, 2015. Available online: https://snu-primo.hosted.exlibrisgroup.com/permalink/f/1l6eo7m/82SNU_SSPACE210371/121219 (accessed on 13 February 2015).
- Bae, J.A.; Bae, W.K.; Kim, S.J.; Ko, Y.S.; Kim, K.Y.; Park, S.Y.; Yu, Y.H.; Kim, E.A.; Chung, I.J.; Kim, H.; et al. A new KSRP-binding compound suppresses distant metastasis of colorectal cancer by targeting the oncogenic KITENIN complex. Mol. Cancer 2021, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.H.; Mun, S.K.; Kim, J.J.; Chang, D.J.; Yee, S.T. Anti-inflammatory effects of Phlebia sp. extract in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biomed. Res. Int. 2022, 2022, 2717196. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, Y.; Dalci, K.; Karakas, H.E.; Erbil-Bilir, S.; Yalav, O.; Sakman, G.; Celik, F.; Arikan, S.; Zeybek, U.; Ergin, M.; et al. Tumor-derived CTF1 (Cardiotrophin 1) is a critical mediator of stroma-assisted and autophagy-dependent breast cancer cell migration, invasion and metastasis. Autophagy 2023, 19, 306–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Nam, K.I.; Kim, K.K.; Koh, J.T.; Kim, N. Bifunctional role of CrkL during bone remodeling. Int. J. Mol. Sci. 2021, 22, 7007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yang, Y.; Park, S.Y.; Nguyen, T.T.; Seo, Y.W.; Lee, K.H.; Lee, J.H.; Kim, K.K.; Hur, J.S.; Kim, H. The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis. Sci. Rep. 2023, 13, 8136. [Google Scholar] [CrossRef] [PubMed]
- Urai, Y.; Yamawaki, M.; Watanabe, N.; Seki, Y.; Morimoto, T.; Tago, K.; Homma, K.; Sakagami, H.; Miyamoto, Y.; Yamauchi, J. Pull down assay for GTP-bound form of sar1a reveals its activation during morphological differentiation. Biochem. Biophys. Res. Commun. 2018, 503, 2047–2053. [Google Scholar] [CrossRef]









| No. | δC,1 type | δH, (J in Hz) 1,2 | COSY | HMBC |
|---|---|---|---|---|
| 1 | 115.4, C | |||
| 2 | 147.0, C | |||
| 2-NHMe | 30.0, CH3 | 2.96, s | 2 | |
| 3 | 112.0, CH | 6.74, d (7.8) | 4 | |
| 4 | 134.2, CH | 7.42, dd (7.8, 1.3) | 3 | |
| 5 | 130.8, C | |||
| 6 | 131.3, CH | 7.76, d (1.3) | 2, 4, 5, 2′ | |
| 2′ | 171.7, C | |||
| 4′ | 123.3, CH | 8.07, s | 2′, 5′, 6′ | |
| 5′ | 150.6, C | |||
| 6′ | 165.6, C | |||
| 2″ | 123.1, CH | 7.27, s | 3″, 4″, 5″ | |
| 3″ | 117.8, C | |||
| 4″ | 128.2, C | |||
| 5″ | 137.9, C | |||
| 6″ | 112.2, CH | 7.32, d (7.8) | 7″ | |
| 7″ | 122.3, CH | 7.04, dd (7.8, 7.8) | 6″, 8″ | |
| 8″ | 119.5, CH | 6.94, dd (7.8, 7.8) | 7″, 9″ | |
| 9″ | 119.8, CH | 7.48, d (7.8) | 8″ | |
| 10″ | 45.6, CH | 4.27, d (7.0) | 11″ | 4, 5, 4′, 3″, 4″ |
| 11″ | 75.6, CH | 4.41, ddd (7.0, 7.0, 4.2) | 10″, 12″ | |
| 12″ | 66.2, CH2 | 3.61 d (7.0, 4.2) 3.48 d (7.0, 4.2) | 11″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulat, S.; Yang, I.; Lee, J.; Hwang, S.; Zhou, R.; Gamage, C.D.B.; Varlı, M.; Taş, İ.; Yang, Y.; Park, S.-Y.; et al. Anithiactin D, a Phenylthiazole Natural Product from Mudflat-Derived Streptomyces sp., Suppresses Motility of Cancer Cells. Mar. Drugs 2024, 22, 88. https://doi.org/10.3390/md22020088
Pulat S, Yang I, Lee J, Hwang S, Zhou R, Gamage CDB, Varlı M, Taş İ, Yang Y, Park S-Y, et al. Anithiactin D, a Phenylthiazole Natural Product from Mudflat-Derived Streptomyces sp., Suppresses Motility of Cancer Cells. Marine Drugs. 2024; 22(2):88. https://doi.org/10.3390/md22020088
Chicago/Turabian StylePulat, Sultan, Inho Yang, Jihye Lee, Sunghoon Hwang, Rui Zhou, Chathurika D. B. Gamage, Mücahit Varlı, İsa Taş, Yi Yang, So-Yeon Park, and et al. 2024. "Anithiactin D, a Phenylthiazole Natural Product from Mudflat-Derived Streptomyces sp., Suppresses Motility of Cancer Cells" Marine Drugs 22, no. 2: 88. https://doi.org/10.3390/md22020088
APA StylePulat, S., Yang, I., Lee, J., Hwang, S., Zhou, R., Gamage, C. D. B., Varlı, M., Taş, İ., Yang, Y., Park, S. -Y., Hong, A., Kim, J. -H., Oh, D. -C., Kim, H., Nam, S. -J., & Kang, H. (2024). Anithiactin D, a Phenylthiazole Natural Product from Mudflat-Derived Streptomyces sp., Suppresses Motility of Cancer Cells. Marine Drugs, 22(2), 88. https://doi.org/10.3390/md22020088