Sterilization of Exopolysaccharides Produced by Deep-Sea Bacteria: Impact on Their Stability and Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Rheological and Viscous Properties of Polysaccharides
2.2. Molecular Weight and Polydispersity of Polysaccharides
2.3. Chemical Characterization
2.4. Sterility of Polysaccharides
2.5. Toxicity of Polysaccharides
3. Experimental
3.1. Materials
3.2. Sterilization Procedures
3.2.1. Ethylene Oxide
3.2.2. Radiation
3.2.3. Cold Plasmas
3.3. Characterization of Marine Polysaccharides
3.3.1. Steady Shear and Dynamic Oscillatory Measurements
3.3.2. Molecular Weight by SEC/MALLS Analyses
3.3.3. Electrophoretic Mobility
3.3.4. Fourier Transform Infrared (FTIR) Analyses
3.3.5. Microbiology
3.3.6. Cytotoxicity of Ethylene Oxide Residues
3.3.7. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Shriver, Z; Raguram, S; Sasisekharan, R. Glycomics: A pathway to a class of new and improved therapeutics. Nat Rev Drug Discov 2004, 3, 863–873. [Google Scholar]
- Ernst, B; Magnani, JL. From carbohydrate leads to glycomimetic drugs. Nat Rev Drug Discov 2009, 8, 661–677. [Google Scholar]
- Flemming, H-C; Wingender, J. The biofilm matrix. Nat Rev Micro 2010, 8, 623–633. [Google Scholar]
- Ryder, C; Byrd, M; Wozniak, DJ. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr Opin Microbiol 2007, 10, 644–648. [Google Scholar]
- Saravanan, P; Jayachandran, S. Preliminary characterization of exopolysaccharides produced by a marine biofilm-forming bacterium Pseudoalteromonas ruthenica (SBT 033). Lett Appl Microbiol 2008, 46, 1–6. [Google Scholar]
- Sutherland, IW. Extracellular Polysaccharides. In Biotechnology, 2nd ed; Rehm, HJ, Reed, G, Eds.; VCH: Weinheim, Germany, 1996; pp. 613–657. [Google Scholar]
- Sutherland, IW. Novel and established applications of microbial polysaccharides. Trends Biotechnol 1998, 16, 41–46. [Google Scholar]
- Rehm, BHA. Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 2010, 8, 578–592. [Google Scholar]
- Bhaskar, PV; Bhosle, NB. Microbial extracellular polymeric substances in marine biogeochemical processes. Curr Sci 2005, 88, 45–53. [Google Scholar]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: an overview. Mar Drugs 2010, 8, 2435–2465. [Google Scholar]
- d’Ayala, GG; Malinconico, M; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar]
- Nichols Mancuso, CA; Garon, S; Bowman, JP; Raguenes, G; Guezennec, J. Production of exopolysaccharides by Antarctic marine bacterial isolates. J Appl Microbiol 2004, 96, 1057–1066. [Google Scholar]
- Nichols Mancuso, CA; Guezennec, J; Bowman, JP. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar Biotechnol 2005, 7, 253–271. [Google Scholar]
- Arena, A; Gugliandolo, C; Stassi, G; Pavone, B; Iannello, D; Bisignano, G; Maugeri, TL. An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: Antiviral activity on immunocompetent cells. Immunol Lett 2009, 123, 132–137. [Google Scholar]
- Yim, JH; Son, E; Pyo, S; Lee, HK. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Mar Biotechnol 2005, 7, 331–338. [Google Scholar]
- Deming, JW. Deep ocean environmental biotechnology. Curr Opin Biotechnol 1998, 9, 283–287. [Google Scholar]
- Guezennec, J. Deep-sea hydrothermal vents: A new source of innovative bacterial exopolysaccharides of biotechnological interest. J Ind Microbiol Biotechnol 2002, 29, 204–208. [Google Scholar]
- Raguenes, G; Christen, R; Guezennec, J; Pignet, P; Barbier, G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, Alvinella pompejana. Int J Syst Bacteriol 1997, 47, 989–995. [Google Scholar]
- Rougeaux, H; Kervarec, N; Pichon, R; Guezennec, J. Structure of the exopolysaccharide of Vibrio diabolicus isolated from a deep-sea hydrothermal vent. Carbohydr Res 1999, 322, 40–45. [Google Scholar]
- Zanchetta, P; Lagarde, N; Guezennec, J. A new bone-healing material: a hyaluronic acid-like bacterial exopolysaccharide. Calcif Tissue Int 2003, 72, 74–79. [Google Scholar]
- Raguenes, GH; Peres, A; Ruimy, R; Pignet, P; Christen, R; Loaec, M; Rougeaux, H; Barbier, G; Guezennec, JG. Alteromonas infernus sp. nov., a new polysaccharide-producing bacterium isolated from a deep-sea hydrothermal vent. J Appl Microbiol 1997, 82, 422–430. [Google Scholar]
- Roger, O; Kervarec, N; Ratiskol, J; Colliec-Jouault, S; Chevolot, L. Structural studies of the main exopolysaccharide produced by the deep-sea bacterium Alteromonas infernus. Carbohydr Res 2004, 339, 2371–2380. [Google Scholar]
- Colliec Jouault, S; Chevolot, L; Helley, D; Ratiskol, J; Bros, A; Sinquin, C; Roger, O; Fischer, AM. Characterization, chemical modifications and in vitro anticoagulant properties of an exopolysaccharide produced by Alteromonas infernus. Biochim Biophys Acta 2001, 1528, 141–151. [Google Scholar]
- Matou, S; Colliec-Jouault, S; Galy-Fauroux, I; Ratiskol, J; Sinquin, C; Guezennec, J; Fischer, A-M; Helley, D. Effect of an oversulfated exopolysaccharide on angiogenesis induced by fibroblast growth factor-2 or vascular endothelial growth factor in vitro. Biochem Pharmacol 2005, 69, 751–759. [Google Scholar]
- Bindal, A; Narsimhan, G; Hem, SL; Kulshreshtha, A. Structural Changes in Xanthan Gum Solutions During Steam Sterilization for Sterile Preparations. Pharm Dev Technol 2007, 12, 159–167. [Google Scholar]
- Kök, MS; Hill, SE; Mitchell, JR. Viscosity of galactomannans during high temperature processing: influence of degradation and solubilisation. Food Hydrocoll 1999, 13, 535–542. [Google Scholar]
- Moisan, M; Barbeau, J; Moreau, S; Pelletier, J; Tabrizian, M; Yahia, LH. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm 2001, 226, 1–21. [Google Scholar]
- Matthews, KH; Stevens, HNE; Auffret, AD; Humphrey, MJ; Eccleston, GM. Gamma-irradiation of lyophilised wound healing wafers. Int J Pharm 2006, 313, 78–86. [Google Scholar]
- Fatimi, A; François Tassin, J; Quillard, S; Axelos, MAV; Weiss, P. The rheological properties of silated hydroxypropylmethylcellulose tissue engineering matrices. Biomaterials 2008, 29, 533–543. [Google Scholar] [Green Version]
- Chauvelon, G; Doublier, J-L; Buléon, A; Thibault, J-F; Saulnier, L. Rheological properties of sulfoacetate derivatives of cellulose. Carbohydr Res 2003, 338, 751–759. [Google Scholar]
- Kapoor, VP; Taravel, FR; Joseleau, J-P; Milas, M; Chanzy, H; Rinaudo, M. Cassia spectabilis DC seed galactomannan: Structural, crystallographical and rheological studies. Carbohydr Res 1998, 306, 231–241. [Google Scholar]
- Fatimi, A; Tassin, J-F; Turczyn, R; Axelos, MAV; Weiss, P. Gelation studies of a cellulose-based biohydrogel: The influence of pH, temperature and sterilization. Acta Biomater 2009, 5, 3423–3432. [Google Scholar] [Green Version]
- Yoo, S-H; Jane, J-L. Molecular weights and gyration radii of amylopectins determined by high-performance size-exclusion chromatography equipped with multi-angle laser-light scattering and refractive index detectors. Carbohydr Polym 2002, 49, 307–314. [Google Scholar]
- Franz, G; Feuerstein, U. Chemical stability of some model polysaccharides. Macromol Symp 1997, 120, 169–181. [Google Scholar]
- Sintzel, MB; Merkli, A; Tabatabay, C; Gurny, R. Influence of Irradiation Sterilization on Polymers Used as Drug Carriers—A Review. Drug Dev Ind Pharm 1997, 23, 857–878. [Google Scholar]
- Schuetz, YB; Gurny, R; Jordan, O. A novel thermoresponsive hydrogel based on chitosan. Eur J Pharm Biopharm 2008, 68, 19–25. [Google Scholar]
- Andriola Silva Brun-Graeppi, AK; Richard, C; Bessodes, M; Scherman, D; Narita, T; Ducouret, G; Merten, O-W. The effect of sterilization methods on the thermo-gelation properties of xyloglucan hydrogels. Polym Degrad Stab 2010, 95, 254–259. [Google Scholar]
- Laroussi, M; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 2004, 233, 81–86. [Google Scholar]
- Kinnari, TJ; Esteban, J; Zamora, N; Fernandez, R; López-Santos, C; Yubero, F; Mariscal, D; Puertolas, JA; Gomez-Barrena, E. Effect of surface roughness and sterilization on bacterial adherence to ultra-high molecular weight polyethylene. Clin Microbiol Infect 2010, 16, 1036–1041. [Google Scholar]
- Simmons, A; Hyvarinen, J; Poole-Warren, L. The effect of sterilisation on a poly(dimethylsiloxane)/poly(hexamethylene oxide) mixed macrodiol-based polyurethane elastomer. Biomaterials 2006, 27, 4484–4497. [Google Scholar]
- Marreco, PR; da Luz Moreira, P; Genari, SC; Moraes, ÂM. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. J Biomed Mater Res Part B Appl Biomater 2004, 71B, 268–277. [Google Scholar]
- Barbucci, R; Lamponi, S; Borzacchiello, A; Ambrosio, L; Fini, M; Torricelli, P; Giardino, R. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials 2002, 23, 4503–4513. [Google Scholar]
- Silva, R; Elvira, C; Mano, J; San Román, J; Reis, R. Influence of β-radiation sterilisation in properties of new chitosan/soybean protein isolate membranes for guided bone regeneration. J Mater Sci Mater Med 2004, 15, 523–528. [Google Scholar]
- Ellis, JL; Titone, JC; Tomasko, DL; Annabi, N; Dehghani, F. Supercritical CO2 sterilization of ultra-high molecular weight polyethylene. J Supercrit Fluids 2010, 52, 235–240. [Google Scholar]
- Guezennec, J; Pignet, P; Raguenes, G; Rougeaux, H. Ifremer, Institut Français de Recherche pour l’Exploitation de la Mer. Souche bactérienne marine du genre Vibrio, polysaccharides hydrosolubles produits par cette souche, et leurs utilisations. French Patent No FR2760022, EP0975791, WO9838327 1998. [Google Scholar]
- Guezennec, J; Pignet, P; Raguenes, G. Ifremer, Institut Français de Recherche pour l’Exploitation de la Mer. Souche bactérienne marine du genre Alteromonas, exopolysaccharides hydrosolubles produits par cette souche, et leurs utilisations. French Patent No FR 2 755 142 1998. [Google Scholar]
- Cross, MM. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J Colloid Sci 1965, 20, 417–437. [Google Scholar]
- Lee, HG; Cowman, MK. An Agarose Gel Electrophoretic Method for Analysis of Hyaluronan Molecular Weight Distribution. Anal Biochem 1994, 219, 278–287. [Google Scholar]
- Vinatier, C; Magne, D; Weiss, P; Trojani, C; Rochet, N; Carle, GF; Vignes-Colombeix, C; Chadjichristos, C; Galera, P; Daculsi, G; Guicheux, J. A silanized hydroxypropyl methylcellulose hydrogel for the three-dimensional culture of chondrocytes. Biomaterials 2005, 26, 6643–6651. [Google Scholar]





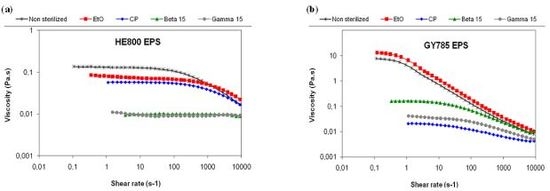
 ) and gamma (
) and gamma (
 ). (a) HE800 EPS produced by Vibrio diabolicus; (b) GY785 EPS produced by Alteromonas infernus.
). (a) HE800 EPS produced by Vibrio diabolicus; (b) GY785 EPS produced by Alteromonas infernus.
 ) and gamma (
) and gamma (
 ). (a) HE800 EPS produced by Vibrio diabolicus; (b) GY785 EPS produced by Alteromonas infernus.
). (a) HE800 EPS produced by Vibrio diabolicus; (b) GY785 EPS produced by Alteromonas infernus.

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rederstorff, E.; Fatimi, A.; Sinquin, C.; Ratiskol, J.; Merceron, C.; Vinatier, C.; Weiss, P.; Colliec-Jouault, S. Sterilization of Exopolysaccharides Produced by Deep-Sea Bacteria: Impact on Their Stability and Degradation. Mar. Drugs 2011, 9, 224-241. https://doi.org/10.3390/md9020224
Rederstorff E, Fatimi A, Sinquin C, Ratiskol J, Merceron C, Vinatier C, Weiss P, Colliec-Jouault S. Sterilization of Exopolysaccharides Produced by Deep-Sea Bacteria: Impact on Their Stability and Degradation. Marine Drugs. 2011; 9(2):224-241. https://doi.org/10.3390/md9020224
Chicago/Turabian StyleRederstorff, Emilie, Ahmed Fatimi, Corinne Sinquin, Jacqueline Ratiskol, Christophe Merceron, Claire Vinatier, Pierre Weiss, and Sylvia Colliec-Jouault. 2011. "Sterilization of Exopolysaccharides Produced by Deep-Sea Bacteria: Impact on Their Stability and Degradation" Marine Drugs 9, no. 2: 224-241. https://doi.org/10.3390/md9020224
APA StyleRederstorff, E., Fatimi, A., Sinquin, C., Ratiskol, J., Merceron, C., Vinatier, C., Weiss, P., & Colliec-Jouault, S. (2011). Sterilization of Exopolysaccharides Produced by Deep-Sea Bacteria: Impact on Their Stability and Degradation. Marine Drugs, 9(2), 224-241. https://doi.org/10.3390/md9020224