2.1. Cultivation and Identification
The marine fungi investigated in this study were predominantly isolated from red seaweed (marine alga). Three strains were isolated from Kappaphycus alvarezii (Doty) Doty ex P.C. Silva (synonym: Kappaphycus cottonii (Weber-van Bosse) Doty ex P.C. Silva), two strains from Eucheuma edule (Kützing) Weber-van Bosse and one strain from Gracilaria sp. Surface sterilization of the algae followed by cultivation using agar media containing antibiotics was the best way to isolate pure strains of fungi.
All marine fungi were cultivated using different culture media to induce sporulation. Finally, some fungi producing spores could be identified by their morphological characteristics. Thus, three isolates were identified as Aspergillus sp. (KT13), Lasiodiplodia theobromae (Pat.) Griffon et Maubl. (KT26) and Epicoccum nigrum Link (KT28). Two algicolous isolates were identified as Xylaria psidii J.D. Rogers & Hemmes (KT30) and Coniothyrium sp. (KT33). Six strains could not be identified, five of which did not form spores under any of the applied culture conditions.
As it is difficult to obtain sexually reproducing forms, molecular biology-based methods such as sequencing rDNA can be a relevant strategy [
6]. The rapid taxonomic assessment of fungal strains might be useful for drug discovery studies based on such organisms because it could reduce the risk of repetitive isolation of known substances. In the future this task will require more attention.
Salinity is one of the environmental factors that affect fungal growth as well as production of secondary metabolites. In an attempt to select the suitable culture conditions for the production of bioactive compounds, the culture medium salinity was varied using marine salt. Results from these tests were taken into account in further cultivation of each strain.
2.2. Biological Activity of Fungal Isolates
2.2.1. Antibacterial Activity against Gram-Positive Bacteria
Crude extracts isolated from culture broth and mycelia were tested for their ability to inhibit growth of human and fish pathogenic bacteria. Most ethyl acetate extracts isolated from culture broth exhibited growth arresting activity against the test organisms. In sharp contrast to the ethyl acetate extracts, ethanol extracts isolated from culture broth as well as dichloromethane, methanol and water extracts from dried mycelia showed no activity. Dichloromethane extracts of KT30 and KT31 displayed an exception to this rule. Thus, further investigations were focused on the ethyl acetate extracts.
Table 1 presents the antibacterial activity of the ethyl acetate extracts of fungal culture broth against the Gram-positive bacteria
Bacillus subtilis and
Staphylococcus aureus. Aspergillus sp. (KT13) was the most active fungus against both bacteria with inhibition zone diameters in the range of 24 to 34 mm followed by strains KT31, KT03, KT19,
Lasiodiplodia theobromae (KT26), and KT29, which were moderately active from both freshwater and seawater cultures.
However, salt concentration influenced the fungal cultures. As shown in
Table 1, ethyl acetate extracts of isolates KT19,
L. theobromae (KT26), and KT29 from seawater cultures showed a higher activity than those from the freshwater cultures and
vice versa for the other strains. Salinity had a significant effect on the activity of ethyl acetate extracts of strain KT15. The extract isolated from seawater culture showed no activity against the test organisms in our test systems.
2.2.2. Antibacterial Activity against Gram-Negative Bacteria
Agar diffusion assays of the ethyl acetate extracts showed that the growth of Gram-positive bacteria was more strongly inhibited than that of Gram-negative bacteria. Two strains, KT15 and
Coniothyrium sp. (KT33), showed no activity against any of the test organisms. As presented in
Table 2, extracts of strain KT19 from both culture conditions possessed antibacterial activity with inhibition zones in the range of 13–16 mm and 8–14 mm against
Escherichia coli and
Pseudomonas aeruginosa, respectively. However, extract of
X. psidii (KT30) cultivated in freshwater medium was in fact the most active against
E. coli and
P. aeruginosa with inhibition zones of 23 and 13 mm, respectively. Some strains (KT03, KT19 and KT29) showed better activity when cultivated in seawater medium.
Remarkable activity was also exhibited by strain KT31. The ethyl acetate extract of isolate KT31 which was cultivated in seawater medium showed weak activity against E. coli with the inhibition zone of 10.6 mm, whereas the extract from freshwater culture was moderately active with the inhibition zone of 18.4 mm.
2.2.3. Antibacterial Activity against Fish Pathogenic Bacteria
The ethyl acetate extracts of the culture broth as well as extracts of the mycelial biomass were tested against the Gram-negative, fish pathogenic bacteria Vibrio anguillarum, Aeromonas salmonicida and Yersinia ruckeri. These three bacteria are known as pathogens for marine and freshwater fish. The diseases caused by these microorganisms are commonly known as vibriosis, furunculosis and enteric red mouth disease, respectively.
Compared to antibacterial activity against
E. coli and
P. aeruginosa, the respective strains showed similar effects against the fish pathogenic bacteria (see
Tables 2 and
3). However, the effects against fish pathogenic bacteria tended to be stronger, particularly against
V. anguillarum. Y. ruckeri was the most resistant fish pathogenic test organism. As can be seen in
Table 3, most of the ethyl acetate extracts isolated from the freshwater fungal culture inhibited the growth of fish pathogenic bacteria.
X. psidii (KT30) exhibited the highest activity observed against the fish pathogenic bacteria tested, followed by strain KT19. However, X. psidii (KT30) was more active when cultivated in freshwater medium, whereas fungal strain KT19 was most productive in salt water medium. Interestingly, in the above-mentioned conditions, these two strains showed similar effects against Y. ruckeri. Moreover, isolates of Aspergillus sp. (KT13), strains KT29 and KT31 exhibited similar activities.
Strains KT19 and KT29 displayed strong antibacterial activity when cultivated in seawater medium. There were no significant effects found for strains KT03 and KT32.
2.2.4. Antifungal Activity against Candida maltosa and Cladosporium cucumerinum
Antifungal activity was observed less often among the extracts tested than antibacterial activity. From the eleven isolates only four strains exhibited antifungal properties against
Candida maltosa (see
Table 4).
Both ethyl acetate and methylene chloride (DCM) extracts of
X. psidii (KT30) and strain KT31 showed antifungal activity, although DCM extracts were less active than the EtOAc extracts. From
Table 4 it can be concluded that again
X. psidii (KT30) was the most active organism against
Candida. Strains KT19 and KT29, cultivated in seawater medium, showed similar activity as against human and fish pathogenic bacteria.
The ethyl acetate extracts of fungal culture broth were tested for their fungicidal properties against the phytopathogenic fungus
Cladosporium cucumerinum. Most of the extracts showed fungicidal effects at 400 μg/78.5 mm
2 spot. The inhibition of each active extract is listed in
Table 5.
2.2.5. Cytotoxic Activity
Table 6 presents the results of cytotoxicity assays against human bladder carcinoma cell line 5637 (ATCC HTB-9).
X. psidii (KT30) and strain KT31 were strongly active in the cytotoxicity assays. Extracts of both strains exhibited IC
50 values of 4 μg/mL and 1.5 μg/mL, respectively. Extracts obtained from seawater culture were less active. In the case of KT 30 and KT31 extracts, IC
50 values were found to be 15 μg/mL and 14 μg/mL. However, these values represent in all cases a comparable high toxicity to many other fungal (and plant) crude extracts. Even though, it was not possible to identify the active principle until now. Further studies to reveal the compounds responsible shall be needed in order to identify the toxic principle in KT30 and KT31.
2.3. Isolation of a New Natural Product
From fungus KT29 one compound (1) was isolated and investigated further to determine its chemical structure. It was obtained as brown crystals by evaporation of methanol solution. The molecular formula of compound 1 was determined to be C12H10O4 by HR-FTICR-MS with the molecular ion peaks at m/z 241.04721 ([M + Na]+) in positive-ion mode and 217.05042 ([M − H]−) in negative-ion mode.
In order to elucidate the compound’s structure, NMR measurements were carried out in CD
3OD. Interpretation of
1H and
13C-NMR spectra confirmed the presence of 12 carbon atoms and 10 proton signals, including methyl and hydroxyl groups. The presence of hydroxyl groups was confirmed by the IR spectrum showing an absorption band at 3439 cm
−1. The IR (KBr) spectrum also exhibits the presence of a carbonyl group (1623 cm
−1), aromatic system (1584 cm
−1) and olefinic bond (1384 cm
−1). The complete structural elucidation of
1 as well as the unambiguous assignment of all
1H and
13C NMR signals was based on 2D NMR experiments (HMBC, HSQC and ROESY). In particular, the attachment of the OH group at C-1 and not at C-2 is based on the HMBC correlation of H-3 with the carbonyl signal at 176.9 ppm (C-11) as well as on the high-field shift of C-9 (117.8 ppm) due to two OR substituents in
ortho position. The
1H and
13C-NMR data of compound
1 are presented in
Table 7.
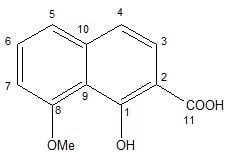
According to the spectroscopic data, compound
1 was determined as 2-carboxy-8-methoxynaphthalene-1-ol (
Figure 1). To date, this compound was only known as an intermediate in the synthesis of a naphthalene carboxylic acid (C
20H
18O
8) using naphthalenediol (C
10H
8O
2) as starting material [
23,
24]. Thus, the discovery of compound
1 as a natural product is new.
In agar diffusion assays, compound
1 showed no antimicrobial activity against
Staphylococcus aureus,
Pseudomonas aeruginosa,
Escherichia coli at 100 μg/disc and
Candida maltosa at 200 μg/disc. Compound
1 was also tested
in vitro against the human bladder carcinoma cell line 5637 with etoposide as the positive control. The results showed that the compound possesses a negligible cytotoxic activity with an IC
50 value of 0.34 mM, compared to etoposide with an IC
50 value of 0.6 μM. Against
Cladosporium cucumerinum, the compound was not active at a concentration of 200 μg/spot. In contrast to our results, many other naphthalene derivatives, mainly naphthoquinones, possess strong antibacterial, antiviral and further activities [
25]. This discrepancy might be due to the following reasons: Compound
1 does not possess a chinoid structure (as do many of the active naphthalene derivatives), and it can be easily metabolized by esterification or etherification of the hydroxyl group at C-1 and the carboxyl group at C-2. The carboxyl group itself renders the compound quite polar and thereby massively reduces transmembrane transport, resulting in a low (cellular) availability.
In addition to the described new substance another isolated compound displayed stronger activity. Unfortunately due to a very low yield the structural elucidation of this compound has not been completed.