Impact of Bisphenol A on the Cardiovascular System — Epidemiological and Experimental Evidence and Molecular Mechanisms
Abstract
:1. Introduction
2. Impact of BPA on the CV System—Epidemiological Studies
3. Impact of BPA on the CV System—Experimental Studies
3.1. Definition of “Low-Dose” BPA in Experimental Studies
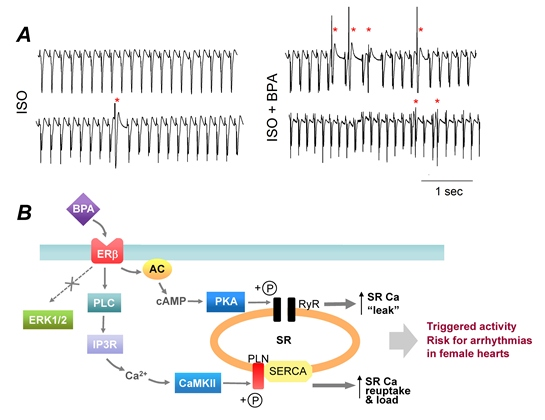
3.2. “Low-Dose” Experimental Studies—Rapid Impacts of BPA on the Heart

3.3. “Low-Dose” Experimental Studies—Effects of Chronic BPA Exposure
3.4. “High-Dose” Experimental Studies
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Dodds, E.C.; Lawson, W. Synthetic oestrogenic agents without the phenanthrene nucleus. Nature 1936, 137. [Google Scholar] [CrossRef]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef]
- Brotons, J.A.; Olea-Serrano, M.F.; Villalobos, M.; Pedraza, V.; Olea, N. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 1995, 103, 608–612. [Google Scholar] [CrossRef]
- Kang, J.H.; Kito, K.; Kondo, F. Factors influencing the migration of bisphenol a from cans. J. Food Prot. 2003, 66, 1444–1447. [Google Scholar]
- Le, H.H.; Carlson, E.M.; Chua, J.P.; Belcher, S.M. Bisphenol a is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008, 176, 149–156. [Google Scholar] [CrossRef]
- Wilson, N.K.; Chuang, J.C.; Morgan, M.K.; Lordo, R.A.; Sheldon, L.S. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-a, and nonylphenol at home and daycare. Environ. Res. 2007, 103, 9–20. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-a and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Kim, K.; Park, H. Association between urinary concentrations of bisphenol a and type 2 diabetes in korean adults: A population-based cross-sectional study. Int. J. Hyg. Environ. Health 2013, 216, 467–471. [Google Scholar] [CrossRef]
- Cantonwine, D.; Meeker, J.D.; Hu, H.; Sanchez, B.N.; Lamadrid-Figueroa, H.; Mercado-Garcia, A.; Fortenberry, G.Z.; Calafat, A.M.; Tellez-Rojo, M.M. Bisphenol a exposure in mexico city and risk of prematurity: A pilot nested case control study. Environ. Health 2010, 9. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol a and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar]
- Itoh, H.; Iwasaki, M.; Hanaoka, T.; Sasaki, H.; Tanaka, T.; Tsugane, S. Urinary bisphenol-a concentration in infertile japanese women and its association with endometriosis: A cross-sectional study. Environ. Health Prev. Med. 2007, 12, 258–264. [Google Scholar] [CrossRef]
- Ning, G.; Bi, Y.; Wang, T.; Xu, M.; Xu, Y.; Huang, Y.; Li, M.; Li, X.; Wang, W.; Chen, Y.; et al. Relationship of urinary bisphenol a concentration to risk for prevalent type 2 diabetes in chinese adults: A cross-sectional analysis. Ann. Intern. Med. 2011, 155, 368–374. [Google Scholar] [CrossRef]
- Gould, J.C.; Leonard, L.S.; Maness, S.C.; Wagner, B.L.; Conner, K.; Zacharewski, T.; Safe, S.; McDonnell, D.P.; Gaido, K.W. Bisphenol a interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998, 142, 203–214. [Google Scholar] [CrossRef]
- Recchia, A.G.; Vivacqua, A.; Gabriele, S.; Carpino, A.; Fasanella, G.; Rago, V.; Bonofiglio, D.; Maggiolini, M. Xenoestrogens and the induction of proliferative effects in breast cancer cells via direct activation of oestrogen receptor alpha. Food Addit. Contam. 2004, 21, 134–144. [Google Scholar] [CrossRef]
- Vivacqua, A.; Recchia, A.G.; Fasanella, G.; Gabriele, S.; Carpino, A.; Rago, V.; Di Gioia, M.L.; Leggio, A.; Bonofiglio, D.; Liguori, A.; et al. The food contaminants bisphenol a and 4-nonylphenol act as agonists for estrogen receptor alpha in mcf7 breast cancer cells. Endocrine 2003, 22, 275–284. [Google Scholar] [CrossRef]
- Pennie, W.D.; Aldridge, T.C.; Brooks, A.N. Differential activation by xenoestrogens of er alpha and er beta when linked to different response elements. J. Endocrinol. 1998, 158, R11–R14. [Google Scholar]
- Sohoni, P.; Sumpter, J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol a as an antagonist. J. Clin. Endocr. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol a action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Quesada, I.; Fuentes, E.; Viso-Leon, M.C.; Soria, B.; Ripoll, C.; Nadal, A. Low doses of the endocrine disruptor bisphenol-a and the native hormone 17beta-estradiol rapidly activate transcription factor creb. FASEB J. 2002, 16, 1671–1673. [Google Scholar]
- Wozniak, A.L.; Bulayeva, N.N.; Watson, C.S. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated ca2+ fluxes and prolactin release in gh3/b6 pituitary tumor cells. Environ. Health Perspect. 2005, 113, 431–439. [Google Scholar] [CrossRef]
- Walsh, D.E.; Dockery, P.; Doolan, C.M. Estrogen receptor independent rapid non-genomic effects of environmental estrogens on [Ca2+]i in human breast cancer cells. Mol. Cell. Endocrinol. 2005, 230, 23–30. [Google Scholar] [CrossRef]
- Zsarnovszky, A.; Le, H.H.; Wang, H.S.; Belcher, S.M. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: Potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol a. Endocrinology 2005, 146, 5388–5396. [Google Scholar] [CrossRef]
- Gao, X.; Liang, Q.; Chen, Y.; Wang, H.-S. Molecular mechanisms underlying the rapid arrhythmogenic action of bisphenol a in female rat hearts. Endocrinology 2013, 154, 4607–4617. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from the endocrine society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of urinary bisphenol a concentration with heart disease: Evidence from nhanes 2003/06. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.T.; Wareham, N.J.; et al. Urinary bisphenol a concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef]
- Melzer, D.; Gates, P.; Osborn, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Schofield, P.; Mosedale, D.; et al. Urinary bisphenol a concentration and angiography-defined coronary artery stenosis. PLoS One 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol a and peripheral arterial disease: Results from the nhanes. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S. Urinary bisphenol a and hypertension in a multiethnic sample of us adults. J. Environ. Public Health 2012, 2012. [Google Scholar] [CrossRef]
- Bae, S.; Kim, J.H.; Lim, Y.H.; Park, H.Y.; Hong, Y.C. Associations of bisphenol a exposure with heart rate variability and blood pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef]
- LaKind, J.S.; Goodman, M.; Naiman, D.Q. Use of nhanes data to link chemical exposures to chronic diseases: A cautionary tale. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Lakind, J.S.; Goodman, M.; Mattison, D.R. Bisphenol a and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: A systematic review of epidemiologic research. Crit. Rev. Toxicol. 2014, 44, 121–150. [Google Scholar] [CrossRef]
- Olsen, L.; Lind, L.; Lind, P.M. Associations between circulating levels of bisphenol a and phthalate metabolites and coronary risk in the elderly. Ecotoxicol. Environ. Saf. 2012, 80, 179–183. [Google Scholar] [CrossRef]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; vom Saal, F.S. In vivo effects of bisphenol a in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Dong, M.; Song, W.; Belcher, S.M.; Wang, H.-S. Bisphenol a and 17b-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Yan, S.; Song, W.; Chen, Y.; Hong, K.; Rubinstein, J.; Wang, H.-S. Low-dose bisphenol a and estrogen increase ventricular arrhythmias following ischemia-reperfusion in female rat hearts. Food Chem. Toxicol. 2013, 56C, 75–80. [Google Scholar]
- Balke, C.W.; Kaplinsky, E.; Michelson, E.L.; Naito, M.; Dreifus, L.S. Reperfusion ventricular tachyarrhythmias: Correlation with antecedent coronary artery occlusion tachyarrhythmias and duration of myocardial ischemia. Am. Heart J. 1981, 101, 449–456. [Google Scholar] [CrossRef]
- Bernier, M.; Manning, A.S.; Hearse, D.J. Reperfusion arrhythmias: Dose-related protection by anti-free radical interventions. Am. J. Physiol. 1989, 256, H1344–H1352. [Google Scholar]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef]
- Myers, J.P.; Zoeller, R.T.; vom Saal, F.S. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ. Health Perspect. 2009, 117, 1652–1655. [Google Scholar]
- Liang, Q.; Gao, X.; Chen, Y.; Hong, K.; Wang, H.-S. Cellular mechanism of the nonmonotonic dose response of bisphenol a in rat cardiac myocytes. Environ. Health Perspect. 2014, 122, 601–608. [Google Scholar]
- Belcher, S.M.; Chen, Y.; Yan, S.; Wang, H.S. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol a. Endocrinology 2012, 153, 712–720. [Google Scholar] [CrossRef]
- Patel, B.B.; Raad, M.; Sebag, I.A.; Chalifour, L.E. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol. Sci. 2013, 133, 174–185. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, M.K.; Kang, G.H.; Lee, K.J.; Choi, S.H.; Lim, S.; Oh, B.C.; Park, D.J.; Park, K.S.; Jang, H.C.; et al. Chronic exposure to bisphenol a can accelerate atherosclerosis in high-fat-fed apolipoprotein e knockout mice. Cardiovasc. Toxicol. 2013, 14, 120–128. [Google Scholar]
- Aboul Ezz, H.S.; Khadrawy, Y.A.; Mourad, I.M. The effect of bisphenol a on some oxidative stress parameters and acetylcholinesterase activity in the heart of male albino rats. Cytotechnology 2013. [Google Scholar] [CrossRef]
- Chapalamadugu, K.C.; Vandevoort, C.A.; Settles, M.L.; Robison, B.D.; Murdoch, G.K. Maternal bisphenol a exposure impacts the fetal heart transcriptome. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Calafat, A.M.; Weuve, J.; Ye, X.; Jia, L.T.; Hu, H.; Ringer, S.; Huttner, K.; Hauser, R. Exposure to bisphenol a and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009, 117, 639–644. [Google Scholar] [CrossRef]
- Wang, F.; Hua, J.; Chen, M.; Xia, Y.; Zhang, Q.; Zhao, R.; Zhou, W.; Zhang, Z.; Wang, B. High urinary bisphenol a concentrations in workers and possible laboratory abnormalities. Occup. Environ. Med. 2012, 69, 679–684. [Google Scholar] [CrossRef]
- Posnack, N.G.; Jaimes, R.; Asfour, H.; Swift, L.M.; Wengrowski, A.M.; Sarvazyan, N.; Kay, M.W. Bisphenol a exposure and cardiac electrical conduction in excised rat hearts. Environ. Health Perspect. 2014, 122, 384–390. [Google Scholar]
- Pant, J.; Ranjan, P.; Deshpande, S.B. Bisphenol a decreases atrial contractility involving no-dependent g-cyclase signaling pathway. J. Appl. Toxicol. 2011, 31, 698–702. [Google Scholar] [CrossRef]
- Chen, S.J.; Li, H.; Durand, J.; Oparil, S.; Chen, Y.F. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation 1996, 93, 577–584. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.F.; Greene, G.L.; Oparil, S.; Thompson, J.A. Estrogen inhibits vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation 1999, 100, 1639–1645. [Google Scholar] [CrossRef]
- Dai-Do, D.; Espinosa, E.; Liu, G.; Rabelink, T.J.; Julmy, F.; Yang, Z.; Mahler, F.; Luscher, T.F. 17 beta-estradiol inhibits proliferation and migration of human vascular smooth muscle cells: Similar effects in cells from postmenopausal females and in males. Cardiovasc. Res. 1996, 32, 980–985. [Google Scholar]
- Pellegrini, M.; Bulzomi, P.; Lecis, M.; Leone, S.; Campesi, I.; Franconi, F.; Marino, M. Endocrine disruptors differently influence estrogen receptor beta and androgen receptor in male and female rat vsmc. J. Cell. Physiol. 2013. [Google Scholar]
- Asano, S.; Tune, J.D.; Dick, G.M. Bisphenol a activates maxi-k (k(ca)1.1) channels in coronary smooth muscle. Br. J. Pharmacol. 2010, 160, 160–170. [Google Scholar] [CrossRef]
- O’Reilly, A.O.; Eberhardt, E.; Weidner, C.; Alzheimer, C.; Wallace, B.A.; Lampert, A. Bisphenol a binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Deutschmann, A.; Hans, M.; Meyer, R.; Haberlein, H.; Swandulla, D. Bisphenol a inhibits voltage-activated Ca(2+) channels in vitro: Mechanisms and structural requirements. Mol. Pharmacol. 2013, 83, 501–511. [Google Scholar] [CrossRef]
- Michaela, P.; Maria, K.; Silvia, H.; L’Ubica, L. Bisphenol a differently inhibits Cav3.1, Cav3.2 and Cav3.3 calcium channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 153–163. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, X.; Wang, H.-S. Impact of Bisphenol A on the Cardiovascular System — Epidemiological and Experimental Evidence and Molecular Mechanisms. Int. J. Environ. Res. Public Health 2014, 11, 8399-8413. https://doi.org/10.3390/ijerph110808399
Gao X, Wang H-S. Impact of Bisphenol A on the Cardiovascular System — Epidemiological and Experimental Evidence and Molecular Mechanisms. International Journal of Environmental Research and Public Health. 2014; 11(8):8399-8413. https://doi.org/10.3390/ijerph110808399
Chicago/Turabian StyleGao, Xiaoqian, and Hong-Sheng Wang. 2014. "Impact of Bisphenol A on the Cardiovascular System — Epidemiological and Experimental Evidence and Molecular Mechanisms" International Journal of Environmental Research and Public Health 11, no. 8: 8399-8413. https://doi.org/10.3390/ijerph110808399
APA StyleGao, X., & Wang, H. -S. (2014). Impact of Bisphenol A on the Cardiovascular System — Epidemiological and Experimental Evidence and Molecular Mechanisms. International Journal of Environmental Research and Public Health, 11(8), 8399-8413. https://doi.org/10.3390/ijerph110808399