Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana
Abstract
:1. Introduction
Study Area
2. Materials and Methods
2.1. Institutional Review Board Approval
2.2. Sample Collection and Analysis
2.3. Data Management
2.4. Well Water Contamination Assessment
2.5. Cumulative Risk Assessment
2.5.1. Non-Carcinogen Risk: Hazard Indices
2.5.2. Carcinogenic Risk: Arsenic and Uranium
2.6. Indian Health Service Well Water Data
2.7. Survey Data
2.8. Interview Data
2.9. Risk Communication and Mitigation
3. Results
3.1. Natural Environment
3.1.1. Inorganic Well Water Contaminants Exceeding EPA Primary Drinking Water Standards
3.1.2. Inorganic Well Water Contaminants Exceeding EPA Secondary Drinking Water Standards
3.1.3. Microbial Well Water Contaminants Exceeding EPA Primary Drinking Water Standards
3.1.4. Inorganic Well Water Contaminants below EPA Primary Drinking Water Standards
3.1.5. Predicting Occurrence of Hazardous Inorganic Contaminants
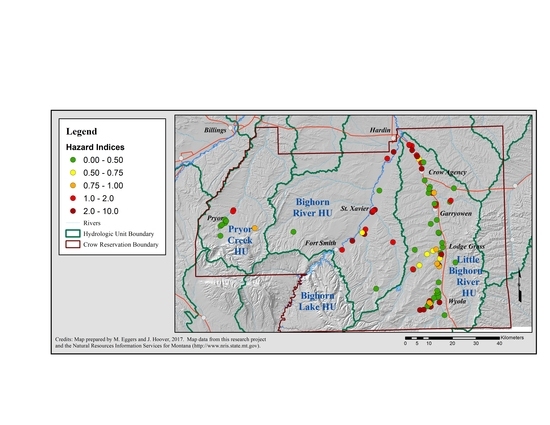
3.1.6. Cumulative Risk Assessment: Non-Carcinogenic Risk
3.1.7. Cumulative Risk Assessment: Carcinogenic Risk
3.1.8. Cumulative Risk Assessment: Non-Carcinogenic Plus Carcinogenic Risk
3.2. Community and Household Factors
3.2.1. Well Water Consumption, TDS and HI
3.2.2. Coping with Poor Quality Well Water
3.2.3. Community Well Stewardship
4. Discussion
4.1. Natural Environment
4.1.1. Primary Contaminants
4.1.2. Secondary Contaminants
4.1.3. Cumulative Risk Assessment Based on Hazard Indices and Slope Factors vs. Summed RQs
4.2. Community and Household Factors
4.2.1. Consumption Data Are Necessary for Assessing Exposures to Well Water Contaminants
4.2.2. Consumption Data Are also Vital to Assessing Population Level Health Risks
4.3. Sources of Contamination
4.3.1. Nitrate
4.3.2. Uranium and Arsenic
4.4. Assessing Vulnerability, Planning Education and Mitigating Risks
4.5. Priority Public Health Issue
- (1)
- Breadth of exposure: 15% of the US population, about 49 million people, rely on home wells;
- (2)
- Nature of exposure: Rural residents consume their well water daily for years, potentially throughout their lives, beginning with pre-natal exposures;
- (3)
- Severity of effects: All four inorganic contaminants most prevalent in Crow home well water—uranium, manganese, arsenic and nitrate—can have severe, lasting health effects on infants and children and can cause disease in adults. The other four most common inorganic contaminants in U.S. well water, radon, boron, fluoride and strontium, represent additional health risks [58,176,177,178].
- (4)
- Likelihood of interactions: The health effects of combinations of these contaminants have not been characterized; interactions as understood in an ecologic framework are nearly entirely unknown.
4.6. Limitations
4.7. Future Research
5. Conclusions
Supplementary Materials
Acknowledgments
- RD83559401-0 and RD83370601-0, National Center for Environmental Research, Science to Achieve Results (NCER STAR), Environmental Protection Agency (EPA).
- FP91674401, STAR Fellowships Research Assistance Agreement Award, EPA.
- 1P50ES026102-01, Center for Native Environmental Health Equity Research, University of New Mexico, Center of Excellence on Environmental Health Disparities Research, National Institute of Environmental Health Sciences, National Institutes of Health (NIH) & EPA.
- P20MD002317, Center for Native Health Partnerships, Montana State University, National Institute of Minority Health and Health Disparities, NIH.
- K12 GM088021 NIGMS ASERT IRACDA postdoctoral fellowship, NIH.
- P20 RR-16455-04, Infrastructure Network for Biomedical Research Excellence (INBRE), National Institute of General Medical Sciences, NIH. Subaward to Little Big Horn College.
- Hopa Mountain, National Science Foundation Division of Earth Sciences, student internships.
Author Contributions
Conflicts of Interest
References
- Balazs, C.L.; Ray, I. The drinking water disparities framework: On the origins and persistence of inequities in exposure. Am. J. Public Health 2014, 104, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Riggs, E.; Hughes, J.; Irvin, D.; Leopard, K. An Overview of Clean Water Access Challenges in the United States; Global Water Challenge and the Environmental Finance Center, School of Government, University of North Carolina: Chapel Hill, NC, USA, 2017; Available online: https://efc.sog.unc.edu/reslib/item/overview-clean-water-access-challenges-united-states (accessed on 7 December 2017).
- Hanna-Attisha, M.; LaChance, J.; Sadler, R.C.; Schnepp, A.C. Elevated blood lead levels in children associated with the Flint drinking water crisis: A spatial analysis of risk and public health response. Am. J. Public Health 2016, 106, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.; Gonzales, M.; Burnette, C.; Benally, M.; Seanez, P.; Shuey, C.; Nez, H.; Nez, C.; Nez, S. Environmental Exposures to Metals in Native Communities and Implications for Child Development: Basis for the Navajo Birth Cohort Study. J. Soc. Work Disabil. Rehabil. 2015, 14, 245–269. [Google Scholar] [CrossRef] [PubMed]
- VanderSlice, J. Drinking water infrastructure and environmental disparities: Evidence and methodological considerations. Am. J. Public Health 2011, 101, S109–S114. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Heaney, C.D.; Cooper, J.; Wilson, O. Built environment issues in unserved and underserved African-American neighborhoods in North Carolina. Environ. Justice 2008, 1, 63–72. [Google Scholar] [CrossRef]
- Balazs, C.; Morello-Frosch, R.; Hubbard, A.; Ray, I. Social disparities in nitrate-contaminated drinking water in California’s San Joaquin Valley. Environ. Health Perspect. 2011, 119, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Balazs, C.L.; Morello-Frosch, R.; Hubbard, A.E.; Ray, I. Environmental justice implications of arsenic contamination in California’s San Joaquin Valley: A cross-sectional, cluster-design examining exposure and compliance in community drinking water systems. Environ. Health 2012, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- BIA (Bureau of Indian Affairs); DOE (Department of Energy); NRC (Nuclear Regulatory Commission); US EPA (Environmental Protection Agency; IHS (Indian Health Service). Health and Environmental Impacts of Uranium Contamination in the Navajo Nation: Five Year Plan. 2008. Available online: http://www.epa.gov/region9/superfund/navajo-nation/pdf/NN-5-Year-Plan-June-12.pdf (accessed on 8 April 2014).
- Centers for Disease Control and Prevention. Keeping Water Safe for the Navajo Nation. 2008. Available online: http://www.cdc.gov/about/pdf/resources/socdc2008.pdf (accessed on 31 March 2014).
- Doyle, J.T.; Kindness, L.; Bear Don’t Walk, U.J.; Realbird, J.; Eggers, M.J.; Crow Environmental Health Steering Committee; Ford, T.E.; Camper, A.K. Addressing disparities in safe drinking water access on the Crow Reservation, Montana. In Proceedings of the Environmental Health Disparities and Environmental Justice Meeting, Raleigh, NC, USA, 29–31 July 2013; p. 28. Available online: https://www.niehs.nih.gov/about/events/pastmtg/assets/docs_c_e/complete_meeting_booklet_508.pdf (accessed on 20 October 2017).
- Firestone, L.; Kaswan, A.; Meraz, S. Environmental justice: Access to clean drinking water. Hast. Law J. 2006, 57, 1367–1386. [Google Scholar]
- Ford, T.E.; Rupp, G.; Butterfield, P.; Camper, A. Protecting Public Health in Small Water Systems: Report of an International Colloquium; Montana Water Center: Bozeman, MT, USA, 2005; p. 52. [Google Scholar]
- Levin, R.B.; Epstein, P.R.; Ford, T.E.; Harrington, W.; Olson, E.; Reichard, E.G. U.S. drinking water challenges in the twenty-first century. Environ. Health Perspect. 2002, 110, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Lewis, L.; Sabogal, R.I.; Bell, C. Survey of unregulated drinking water sources on the Navajo Nation. In Proceedings of the American Public Health Association 137th Annual Meeting and Exposition on Water and Public Health, Philadelphia, PA, USA, 7–11 November 2009; Available online: http://apha.confex.com/apha/137am/webprogram/Paper208881.html (accessed on 1 March 2014).
- Olmstead, S.M. Thirsty colonias: Rate regulation and the provision of water service. Land Econ. 2004, 80, 136–150. [Google Scholar] [CrossRef]
- Parcher, J.W.; Humberson, D.G. CHIPS: A New Way to Monitor Colonias along the United States–Mexico Border; USGS Open-File Report 2007-1230; USGS Enterprise Publishing Network: Rolla, MO, USA, 2010. Available online: http://pubs.usgs.gov/of/2007/1230/ (accessed on 28 March 2014).
- U.S. Department of Agriculture; U.S. Environmental Protection Agency. U.S. Mexico Border Needs Assessment and Support Project; Phase 1 Scoping Assessment Report; USDA, EAP: Washington, DC, USA, 2014. Available online: http://www.rurdev.usda.gov/SupportDocuments/RD_RUS_Phase1ResearchRpt.pdf (accessed on 9 April 2014).
- U.S. Environmental Protection Agency. Environmental Protection Agency. Infrastructure Task Force to Improve Access to Safe Drinking Water and Basic Sanitation in Indian Country. 2013. Available online: http://www.epa.gov/tribal/trprograms/infra-water.htm (accessed on 28 February 2014).
- Villanueava, C.M.; Kogevina, M.; Cordier, S.; Templeton, M.R.; Vermeulen, R.; Nuckols, J.R.; Nieuwenhuijsen, M.J.; Levallois, P. Assessing exposure and health consequences of chemicals in drinking water: Current state of knowledge and research needs. Environ. Health Perspect. 2014, 122, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Heaney, C.D.; Wilson, O. Governance structures and the lack of basic amenities: Can community engagement be effectively used to address environmental injustice in underserved black communities. Environ. Justice 2010, 3, 125–133. [Google Scholar] [CrossRef]
- Backer, L.C.; Tosta, N. Unregulated Drinking Dater Initiative for environmental surveillance and public health. J. Environ. Health 2011, 73, 31–32. [Google Scholar] [PubMed]
- DeSimone, L.A. Quality of Water from Domestic Wells in Principal Aquifers of the United States, 1991–2004; U.S. Geological Survey Scientific Investigations Report 2008-5227; U.S. Geological Survey: Northborough, MA, USA, 2009; p. 139. [Google Scholar]
- Rogan, W.J.; Brady, M.T.; The Committee on Environmental Health and the Committee on Infectious Diseases. American Academy of Pediatrics. Drinking water from private wells and risks to children. Pediatrics 2009, 123, e1123–e1137. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. How EPA Regulates Drinking Water Contaminants. 2017. Available online: https://www.epa.gov/dwregdev/how-epa-regulates-drinking-water-contaminants#make (accessed on 18 May 2017).
- Bischoff, W.E.; Weir, M.; Summers, P.; Haiying, C.; Quandt, S.A.; Liebman, A.K.; Arcury, T.A. The quality of drinking water in North Carolina farmworker camps. Am. J. Public Health 2012, 102, e49–e54. [Google Scholar] [CrossRef] [PubMed]
- Focazio, M.J.; Tipton, D.; Shapiro, S.D.; Geiger, L.H. The chemical quality of self-supplied domestic well water in the United States. Groundw. Monit. Remediat. 2006, 26, 92–104. [Google Scholar] [CrossRef]
- Goss, M.J.; Barry, D.A.J.; Rudolph, D.L. Contamination in Ontario farmstead domestic wells and its association with agriculture: 1. Results from drinking water wells. J. Contam. Hydrol. 1997, 32, 267–293. [Google Scholar] [CrossRef]
- Heaney, C.; Wilson, S.M.; Wilson, O.; Cooper, J. Use of community-owned and managed research to assess the vulnerability of water and sewer services in marginalized and underserved environmental justice communities. J. Environ. Health 2011, 74, 8–17. [Google Scholar] [PubMed]
- Hexemer, A.M.; Pintar, K.; Bird, T.M.; Zentner, S.E.; Garcia, H.P.; Pollari, F. An investigation of bacteriological and chemical water quality and the barriers to private well water sampling in a Southwestern Ontario community. J. Water Health 2008, 6, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.H.; Gonzales, M.; Shuey, C.; Barney, Y.; Lewis, J. Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo Nation, USA. Expo. Health 2017, 9, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, H. Review and Comparison of Regional Groundwater Quality Data in Saskatchewan; Saskatchewan Research Council: Saskatoon, SK, Canada, 2000; p. 37. [Google Scholar]
- Paul, M.P.; Rigrod, P.; Wingate, S.; Borsuk, M.E. A community-driven intervention in Tuftonboro, New Hampshire, succeeds in altering water testing behavior. J. Environ. Health 2015, 78, 30–39. [Google Scholar] [PubMed]
- Robertson, W.; Neil, D. Microbiological quality of drinking water in Canada: An overview of the Health Canada programme. In An Earth Odyssey, Proceedings of the 54th Canadian Geotechnical Conference, Calgary, AB, Canada, 16–19 September 2001; Mahmound, M., Everdingen, R., Eds.; Bitech: Richmond, BC, Canada, 2001; pp. 45–49. [Google Scholar]
- Summers, R.J. Albert a Well Water Survey. A Report Prepared for Alberta Environment; University of Alberta: Edmonton, AB, Canada, 2010. [Google Scholar]
- Walker, M.; Shaw, W.D.; Benson, M. Arsenic consumption and health risk perceptions in a rural western U.S. area. J. Am. Water Resour. Assoc. 2006, 42, 1363–1370. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Uranium. 2012. Available online: http://www.epa.gov/rpdweb00/radionuclides/uranium.html (accessed on 15 April 2014).
- Kurttio, P.; Auvinen, A.; Salonen, L.; Saha, H.; Pekkanen, J.; Makelainen, I.; Vaisanen, S.B.; Penttila, I.M.; Komulainen, H. Renal effects of uranium in drinking water. Environ. Health Perspect. 2002, 110, 337–342. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Uranium in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality. 2012. Available online: http://www.who.int/water_sanitation_health/publications/2012/background_uranium.pdf (accessed on 16 November 2016).
- Kurttio, P.; Komulainen, H.; Leino, A.; Salonen, L.; Auvinen, A.; Saha, H. Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environ. Health Perspect. 2005, 113, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Brugge, D.; De Lemos, J.L.; Oldmixon, B. Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: A review. Rev. Environ. Health 2005, 20, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Brugge, D.; Buchner, V. Health effects of uranium: New research findings. Rev. Environ. Health 2011, 26, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Guallar, E.; Cowie, C.C. Metals in urine and diabetes in U.S. adults. Diabetes 2016, 65, 164–171. [Google Scholar] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Uranium; ATSDR: Atlanta, GA, USA, 2013. [Google Scholar]
- Barlow, P.J. A pilot study on the metal levels in the hair of hyperactive children. Med. Hypotheses 1983, 11, 309–318. [Google Scholar] [CrossRef]
- Bhang, S.-Y.; Cho, S.-C.; Kim, J.-W.; Hong, J.-C.; Shin, M.-S.; Yoo, H.J.; Cho, I.H.; Kim, Y.; Kim, B.N. Relationship between blood manganese levels and children’s attention, cognition, behavior, and academic performance—A nationwide cross-sectional study. Environ. Res. 2013, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.; Laforest, F.; Vandelac, L.; Bellinger, D.; Mergler, D. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ. Health Perspect. 2007, 115, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, M.F.; Sauve, S.; Barbeau, B.; Legrand, M.; Brodeur, M.-È.; Bouffard, T.; Limoges, E.; Bellinger, D.C.; Mergler, D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ. Health Perspect. 2011, 119, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Collipp, P.J.; Chen, S.Y.; Maitinsky, S. Manganese in infant formulas and learning disability. Ann. Nutr. Metab. 1983, 27, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ericson, J.E.; Crinella, F.M.; Clarke-Stewart, K.A.; Allhusen, V.D.; Chan, T.; Robert, R.T. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol. Teratol. 2007, 29, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Henn, B.C.; Schnaas, L.; Ettinger, A.S.; Schwartz, J.; Lamadrid-Figueroa, H.; Hernàndez-Avila, M.; Amarasiriwardena, C.; Hu, H.; Bellinger, D.C.; Wright, R.O.; et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect. 2011, 120, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes-Filho, J.A.; Novaes Cde, O.; Moreira, J.C.; Sarcinelli, P.N.; Mergler, D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ. Res. 2011, 111, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Riojas-Rodríguez, H.; Solís-Vivanco, R.; Schilmann, A.; Montes, S.; Rodríguez, S.; Ríos, C.; Rodríguez-Agudelo, Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ. Health Perspect. 2010, 118, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Levy, D.; Factor-Litvak, P.; Kline, J.; van Geen, A.; Slavkovich, V.; LoIacono, N.J.; et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006, 114, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Zoni, S.; Lucchini, R.G. Manganese exposure: Cognitive, motor and behavioral effects on children: A review of recent findings. Curr. Opin. Pediatr. 2013, 25, 255–260. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Drinking Water Health Advisory for Manganese; EPA: Washington, DC, USA, 2004. Available online: https://www.epa.gov/sites/production/files/2014-09/documents/support_cc1_ magnese_dwreport_0.pdf (accessed on 24 August 2016).
- Hafeman, D.; Factor-Litvak, P.; Cheng, Z.; van Geen, A.; Ahsan, H. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ. Health Perspect. 2007, 115, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Radon; ATSDR: Atlanta, GA, USA, 2012. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp145.pdf (accessed on 28 May 2017).
- Frisbie, S.H.; Mitchell, E.J.; Dustin, H.; Maynard, D.M.; Sarkar, B. World Health Organization Discontinues Its Drinking-Water Guideline for Manganese. Environ. Health Perspect. 2012, 120, 775–778. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. Health Based Screening Levels for Evaluating Water Quality Data. 2014. Available online: https://cida.usgs.gov/hbsl/apex/f?p=104:1:0::NO:RP (accessed on 11 January 2017).
- U.S. Environmental Protection Agency. Nitrates and Nitrites: Toxicity and Exposure Assessment for Children’s Health (TEACH) Chemical Summary. 2007. Available online: http://www.epa.gov/teach/teachsummaries.html (accessed on 1 April 2014).
- Brender, J.D.; Weyer, P.J.; Romitti, P.A.; Mohanty, B.P.; Shinde, M.U.; Vuong, A.M.; Sharkey, J.R.; Dwivedi, D.; Horel, S.A.; Kantamneni, J.; et al. National Birth Defects Prevention Study. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the National Birth Defects Prevention Study. Environ. Health Perspect. 2013, 121, 1083–1089. [Google Scholar] [PubMed]
- Ward, M.H.; deKok, T.M.; Levallois, P.; Brender, J.; Gulis, G.; Nolan, B.T.; VanderSlice, J. Workgroup report: Drinking-water nitrate and health—Recent findings and research needs. Environ. Health Perspect. 2005, 113, 1607–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Arsenic; ATSDR: Atlanta, GA, USA, 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf (accessed on 15 May 2014).
- National Research Council. Critical Aspects of EPA’s IRIS Assessment of Inorganic Arsenic: Interim Report; The National Academies Press: Washington, DC, USA, 2014.
- Gosse, J.A.; Taylor, V.F.; Jackson, B.P.; Hamilton, J.W.; Bodwell, J.E. Monomethylated trivalent arsenic species disrupt steroid receptor interactions with their DNA response elements at non-cytotoxic cellular concentrations. J. Appl. Toxicol. 2014, 34, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Gupta, P.; Ghosh, S.; Mukherjee, S.; Chakraborty, P.; Chatterji, U.; Chattopadhyay, S. Arsenic-induced dose-dependent modulation of the NF-κB/IL-6 axis in thymocytes triggers differential immune responses. Toxicology 2016, 357–358, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Dangleben, N.L.; Skibola, C.F.; Smith, M.T. Arsenic immunotoxicity: A review. Environ. Health 2013, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Keil, D.; Buck, B.; Goossens, D.; Teng, Y.; Spencer, M.; Murphy, L.; Pollard, J.; Eggers, M.; McLaurin, B.; Gerads, R.; et al. Immunotoxicological and neurotoxicological profile of health effects following subacute exposure to geogenic dust from sand dunes at the Nellis Dunes Recreation Area, Las Vegas, NV. Toxicol. Appl. Pharmacol. 2016, 291, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maull, E.A.; Ahsan, H.; Edwards, J.; Longnecker, M.P.; Navas-Acien, A.; Pi, J.; Silbergeld, E.K.; Styblo, M.; Tseng, C.H.; Thayer, K.A.; et al. Evaluation of the associations between arsenic and diabetes: A National Toxicology Program workshop review. Environ. Health Perspect. 2012, 120, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, G.A.; Liu, X.; Loiacono, N.J.; Kline, J.; Factor-Litvak, P.; van Geen, A.; Mey, J.L.; Levey, D.; Abramson, R.; Schwartz, A.; et al. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ. Health 2014, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Carlin, D.; Naujokas, M.F.; Bradham, K.D.; Cowden, J.; Heacock, M.; Henry, H.F.; Lee, J.S.; Thomas, D.J.; Thompson, C.; Tokar, E.J.; et al. Arsenic and environmental health: State of the science and future research opportunities. Environ. Health Perspect. 2016, 124, 890–899. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Drinking water from private wells and risks to children. Pediatrics 2009, 123, 1599–1605. [Google Scholar]
- Postma, J.; Butterfield, P.W.; Odom-Maryon, T.; Hill, W.; Butterfield, P.G. Rural children’s exposures to well water contaminants: Implications in light of the American Academy of Pediatrics’ recent policy statement. J. Am. Acad. Nurse Pract. 2011, 23, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.Q.; Dewey, C.E.; Dore, K.; Majowicz, S.E.; McEwen, S.A.; David, W-T.; Eric, M.; Carr, D.J.; Henson, S.J. Public perceptions of drinking water: A postal survey of residents with private water supplies. BMC Public Health 2006, 6, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreutzwiser, R.; de Loe, R.; Imgrund, K.; Conboy, M.J.; Simpson, H.; Plummer, R. Understanding stewardship behavior: Factors facilitating and constraining private water well stewardship. J. Environ. Manag. 2011, 92, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.A.; Sexton, K. If cumulative risk assessment is the answer, what is the question? Environ. Health Perspect. 2007, 115, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Ryker, S.J.; Small, M.J. Combining occurrence and toxicity information to identify priorities for drinking-water mixture research. Risk Anal. 2008, 28, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Putzrath, R.M. Reducing uncertainty of risk estimates for mixtures of chemicals within regulatory constraints. Regul. Toxicol. Pharmacol. 2000, 31, 44–52. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Framework for Cumulative Risk Assessment; EPA/630/P-02/001F; EPA: Washington, DC, USA, 2003.
- Brulle, R.J.; Pellow, D.N. Environmental Justice: Human health and environmental inequalities. Annu. Rev. Public Health 2006, 27, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Gee, G.C.; Payne-Sturges, D.C. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ. Health Perspect. 2004, 112, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Mohai, P.; Pellow, D.; Roberts, J.T. Environmental justice. Annu. Rev. Environ. Resour. 2009, 34, 405–420. [Google Scholar] [CrossRef]
- Soobader, M.; Cubbin, C.; Gee, G.C.; Rosenbaum, A.; Laurenson, J. Levels of analysis for the study of environmental health disparities. Environ. Res. 2006, 102, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M. An ecologic framework to study and address environmental justice and community health issues. Environ. Justice 2009, 2, 15–23. [Google Scholar] [CrossRef]
- National Institute of Environmental Health Sciences. Advancing Science, Improving Health: A Plan for Environmental Health Research, National Institute of Environmental Health Sciences Strategic Plan 2012–2017; NIH Publication No. 12-7935; National Institutes of Health, U.S. Department of Health and Human Services: Bethesda, MD, USA, 2012; 28p.
- Anderson, B.E.; Naujokas, M.F.; Suk, W.A. Interweaving knowledge resources to address complex environmental health challenges. Environ. Health Perspect. 2015, 123, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Collman, G.W. Community-based approaches to environmental health research around the globe. Rev. Environ. Health 2014, 29, 125–128. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. A Decade of Tribal Environmental Research: Results and Impacts from EPA’s Extramural Grants and Fellowship Programs; Tribal Environmental Health Research Program, NCER, ORD, EPA: Washington, DC, USA, 2004. Available online: http://epa.gov/ncer/tribalresearch/news/results-impacts-010714.pdf (accessed on 12 February 2014).
- Finn, S.; Collman, G. The pivotal role of the social sciences in environmental health sciences research. New Solut. 2016, 26, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Finn, S.; Thompson, C. Community-based participatory research through the lens of environmental health: More than a catchy sounding name. In Proceedings of the International Society for Environmental Epidemiology Conference, Columbia, SC, USA, 26–30 August 2012; Available online: http://journals.lww.com/epidem/Citation/2012/09001/S_002__Community_Based_Participatory_Research.257.aspx (accessed on 13 February 2017).
- Hoover, E.; Renauld, M.; Edelstein, M.R.; Brown, P. Social science collaboration with environmental health. Environ. Health Perspect. 2015, 123, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- McOliver, C.; Camper, A.K.; Doyle, J.T.; Eggers, M.J.; Ford, T.E.; Lila, M.A.; Berner, J.; Campbell, L.; Donatuto, J. Community-based research as a mechanism to reduce environmental health disparities in American Indian and Alaska Native communities. Int. J. Environ. Res. Public Health 2015, 12, 4076–4100. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, L.R.; Dearry, A. Community-based participatory research as a tool to advance environmental health sciences. Environ. Health Perspect. 2002, 110, 155–159. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, L.R.; Finn, S. Citizen Science and Community-Engaged Research in Environmental Public Health. Lab Matters, Association of Public Health Laboratories. Issue ID 279800, 5. Available online: digital.aphl.org/publication/?i=279800#{"issue_id":279800,"page":6} (accessed on 6 May 2016).
- Parker, E.A.; Baldwin, G.T.; Israel, B.; Salinas, M.A. Application of health promotion theories and models for environmental health. Health Educ. Behav. 2004, 31, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Eggers, M.J. Community Based Risk Assessment of Exposure to Waterborne Contaminants on the Crow Reservation, Montana. Ph.D. Thesis, Montana State University, Bozeman, MT, USA, 2014. Available online: http://scholarworks.montana.edu/xmlui/bitstream/handle/1/9358/EggersM0814 (accessed on 6 May 2016).
- Eggers, M.J.; Moore-Nall, A.L.; Doyle, J.T.; Lefthand, M.J.; Young, S.L.; Bends, A.L.; Crow Environmental Health Steering Committee; Camper, A.K. Potential health risks from uranium in home well water: An investigation by the Apsaalooke (Crow) tribal research group. Geosciences 2015, 5, 67–94. [Google Scholar] [CrossRef]
- Martin, C.; Simonds, V.; Lefthand, M.L.; Doyle, J.T.; Eggers, M.J.; Young, S. Perceptions of safe water, Crow Water Quality Project. In Proceedings of the 2017 UCOWR/NIWR Annual Conference “Water in a Changing Environment”, Fort Collins, CO, USA, 13–15 June 2017; p. 129. Available online: http://www.ucowr.org/files/2017_Conference/proceedings/2017_Conference_Proceedings_062717.pdf (accessed on 20 October 2017).
- Martin, C.; Simonds, V.; Eggers, M.J.; Young, S.L.; Lefthand, M.J.; Doyle, J.T. Perceptions of Safe Water: Tribal Residents’ Concerns about Water Quality. 2018; In preparation. [Google Scholar]
- Montana State Governor’s Office of Indian Affairs. Crow Nation. 2013. Available online: https://tribalnations.mt.gov/crow (accessed on 6 June 2017).
- Cummins, C.; Doyle, J.T.; Kindness, L.; Lefthand, M.J.; Bear Don’t Walk, U.J.; Bends, A.; Broadaway, S.C.; Camper, A.K.; Fitch, R.; Ford, T.E.; et al. Community-based participatory research in Indian Country: Improving health through water quality research and awareness. Fam. Community Health 2010, 33, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.T.; Lefthand, M.L.; Young, S.L.; Small, D.; Lefthand, F. Everything Has Water Flowing through It. Environmental Health News. 2016. Available online: http://www.environmentalhealthnews.org/ehs/news/2016/tribal-series/sacred-water (accessed on 24 August 2016).
- Lefthand, M.J.; Eggers, M.J.; Old Coyote, T.J.; Doyle, J.T.; Kindness, L.; Bear Don’t Walk, U.J.; Young, S.L.; Bends, A.L.; Good Luck, B.; Stewart, R.; et al. Holistic community based risk assessment of exposure to contaminants via water sources. In Proceedings of the American Public Health Association Conference, San Francisco, CA, USA, 27–31 October 2012; Available online: http://dissem.in/p/39724405/holistic-community-based-risk-assessment-of-exposure-to-contaminants-via-water-sources (accessed on 6 June 2017).
- Mark, D.; Byron, R. Big Horn Valley Health Center Program Narrative; Bighorn Valley Health Center (BVHC): Hardin, MT, USA, 2010. [Google Scholar]
- Bends, A.; Crow Tribe, Crow Agency, MT, USA. Survey of the Health Needs of the Crow Reservation. Unpublished work. 2015. [Google Scholar]
- Montana Department of Public Health and Human Services. Big Horn County Health Profile. 2015. Available online: http://dphhs.mt.gov/publichealth/Publications/CountyHealthProfiles (accessed on 4 November 2017).
- Montana Department of Public Health and Human Services. Big Horn County Health Profile. 2006. Available online: http://www.docstoc.com/docs/86526062/2006-Montana-County-Health-Profiles—Department-of-Public-Health (accessed on 2 September 2013).
- Eggers, M.J. Community-Based Risk Assessment of Exposure to Waterborne Contaminants, Crow Reservation, Montana. In Proceedings of the Environmental Health Disparities and Environmental Justice Meeting, Raleigh, NC, USA, 29–31 July 2013; pp. 37–38. Available online: https://www.niehs.nih.gov/about/events/pastmtg/assets/docs_c_e/complete_meeting_booklet_508.pdf (accessed on 20 October 2017).
- DuFault, R. In another country: Indian County Environmental Hazard Assessment Training Project Seeks IH Instructors and Mentors. Synergist 2005, 43–47. Available online: https://www.academia.edu/6046645/Volunteering_for_ICEHAP (accessed on 4 November 2017).
- American Indian Higher Education Consortium. Indian Country Environmental Hazard Assessment Program: An Online Training Program Developed for Indigenous People and Government Employees. 2015. Available online: http://www.aihec.org/what-we-do/docs/announce/2015_ICHAP.pdf (accessed on 4 November 2017).
- Tuck, L. Ground-Water Resources along the Little Bighorn River, Crow Indian Reservation, Montana; Water-Resources Investigations Report 03-4052; U.S. Department of the Interior and The U.S. Geological Survey: Helena, MT, USA, 2003; p. 54. [Google Scholar]
- Doyle, J.T.; Redsteer, M.H.; Eggers, M.J. Exploring effects of climate change on Northern Plains American Indian health. Clim. Chang. 2013, 120, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Hamner, S.; Broadaway, S.C.; Berg, E.; Stettner, S.; Pyle, B.H.; Big Man, N.; Old Elk, J.; Eggers, M.J.; Doyle, J.; Kindness, L.; et al. Detection and source tracking of Escherichia coli, harboring intimin and Shiga toxin genes, isolated from the Little Bighorn River, Montana. Int. J. Environ. Health Res. 2013, 24, 341–362. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Broadaway, S.C.; Eggers, M.J.; Doyle, J.T.; Pyle, B.H.; Camper, A.K.; Ford, T.E. Detection of pathogenic and non-pathogenic bacteria in drinking water and associated biofilms on the Crow Reservation, Montana, USA. Microb. Ecol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Israel, B.A.; Eng, E.; Schulz, A.J.; Parker, E.A. Methods in Community-Based Participatory Research for Health; Jossey-Bass, A Wiley Imprint: San Francisco, CA, USA, 2005. [Google Scholar]
- Minkler, M.; Wallerstein, N. Community-Based Participatory Research for Health: From Process to Outcomes; Jossey-Bass, A Wiley Imprint: San Francisco, CA, USA, 2008. [Google Scholar]
- Riederer, A.M.; Thompson, K.M.; Fuentes, J.M.; Ford, T.E. Body weight and water ingestion estimates for women in two communities in the Philippines: The importance of collecting site-specific data. Int. J. Hyg. Environ. Health 2006, 209, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Energy Laboratories. Quality Assurance Manual; Energy Laboratories: Billings, MT, USA, 2015; Available online: https://www.energylab.com/wp-content/uploads/2012/04/Billings-QA-Manual-2015_final1.pdf (accessed on 24 August 2016).
- Montana Department of Environmental Quality. Uranium in Drinking Water. 2009. Available online: http://deq.mt.gov/wqinfo/swp/Guidance.mcpx (accessed on 6 August 2013).
- Pippin, W.; Energy Laboratories, Billings, MT, USA. Personal communication. 2015. [Google Scholar]
- Moore-Nall, A.L. Structural Controls and Chemical Characterization of Brecciation and Uranium Vanadium Mineralization in the Northern Bighorn Basin. Ph.D. Thesis, Montana State University, Bozeman, MT, USA, 2016. [Google Scholar]
- Moore-Nall, A.L.; Lageson, D.R. Uranium vanadium mineralization in Mississippian aged paleokarst, northern Bighorn Basin, Montana and Wyoming, indicates a hydrothermal Permian Phosphoria Formation source of metals including REE and TL. In Proceedings of the Geological Society of America Conference, Denver, CO, USA, 25–28 September 2016; Available online: https://gsa.confex.com/gsa/2016AM/webprogram/Paper287959.html (accessed on 23 January 2017).
- U.S. Census Bureau. Montana Locations by per Capita Income. 2010. Available online: http://en.wikipedia.org/wiki/Montana_locations_by_per_capita_income (accessed on 2 April 2014).
- Zandbergen, P.A. Ensuring confidentiality of geocoded health data: Assessing geographic masking strategies for individual-level data. Adv. Med. 2014, 14, 567049. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.E.; Fetters, T.L. Confidentiality concerns with mapping survey data in reproductive health research. Stud. Fam. Plan. 2007, 38, 309–321. [Google Scholar] [CrossRef]
- Hampton, K.H.; Fitch, M.K.; Allshouse, W.B.; Doherty, I.A.; Gesink, D.C.; Leone, P.A.; Serre, M.L.; Miller, W.C. Mapping health data: Improved privacy protection with donut method geomasking. Am. J. Epidemiol. 2010, 172, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Allshouse, W.B.; Fitch, M.K.; Hampton, K.H.; Gesink, D.C.; Doherty, I.A.; Leone, P.A.; Serre, M.L.; Miller, W.C. Geomasking sensitive health data and privacy protection: An evaluation using an E911 database. Geocarto Int. 2010, 25, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.M.; Peterson, R.K.D.; Montagne, C.; Inskeep, W.P.; Schleier, J.J., III. A dietary risk assessment for indigenous consumption of natural salt deposits in the Darhad Valley, Northern Mongolia. Hum. Ecol. Risk Assess. 2009, 15, 907–922. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Guidelines for Exposure Assessment. 1992. Available online: https://www.epa.gov/irsk/guidelines-exposure-assessment (accessed on 7 December 2017).
- U.S. Environmental Protection Agency. Reference Dose (RfD): Description and Use in Health Risk Assessments Background Document 1A. 1993. Available online: https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments (accessed on 27 November 2017).
- Wongsasuluk, P.; Chotpantara, S.; Siriwong, W.; Robson, M. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ. Geochem. Health 2013, 36, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency, National Center for Environmental Assessment, Integrated Risk Information System (IRIS). IRIS Assessments. Available online: https://www.epa.gov/iris (accessed on 2 December 2017).
- Oak Ridge National Laboratory Risk Assessment Information System. Toxicity Profiles: RAGs A Format for Manganese—CAS Number 7439965. Available online: https://rais.ornl.gov/profiles/mn_ragsa.html (accessed on 2 December 2017).
- U.S. Environmental Protection Agency; Environmental Protection Agency. Environmental Manganese: Guideline Exposure Levels, Evidence of Health Effects and Research Needs. 2005. Available online: https://cfpub.epa.gove/si/si_public_recrod_Report.cfm?dirEntryID=76758 (accessed on 4 December 2017).
- U.S. Environmental Protection Agency, Office of Superfund Remediation and Technology Innovation. Considering a Noncancer Oral Reference Dose for Uranium for Superfund Human Health Risk Assessments. 2016. Available online: https://semspub.epa.gov/work/HQ/196808.pdf (accessed on 4 December 2017).
- U.S. Environmental Protection Agency, National Center for Environmental Assessment, Integrated Risk Information System (IRIS). Chemical Assessment Summary. 1995; Arsenic, Inorganic; CASRN 7440-38-2. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0278_summary.pdf (accessed on 2 December 2017).
- Niemi, C. “Acceptable” Risk Levels for Carcinogens: Their History, Current Use, and How They Affect Surface Water Quality Criteria; Policy Forum #3; Department of Ecology, State of Washington: Lacey, WA, USA, 2013; Available online: http://www.tmw-law.com/news-pdf/SWQSPolicyForumRiskLevel%2002-08-213.pdf (accessed on 14 December 2017).
- California State Water Resources Control Board. Review of Maximum Contaminant Levels. 2017. Available online: https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/documents/reviewofmaximumcontaminantlevels-2017.pdf (accessed on 14 December 2017).
- U.S. Environmental Protection Agency, National Center for Environmental Assessment, Integrated Risk Information System (IRIS). Chemical Assessment Summary. 1993; Uranium, Natural; CASRN 7440-61-1. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0259_summary.pdf (accessed on 2 December 2017).
- U.S. Oak Ridge National Laboratory. Provisional Peer Reviewed Toxicity Values for Superfund (PPRTV). Available online: https://hhpprtv.ornl.gov (accessed on 11 December 2017).
- U.S. Environmental Protection Agency. Radionuclide Table: Radionuclide Carcinogenicity—Slope Factors (Federal Guidance Report No. 13 Morbidity Risk Coefficients). 2015. Available online: https://www.epa.gov/sites/production/files/2015-02/documents/heast2_table_4-d2_0401.pdf (accessed on 11 December 2017).
- State of Massachusetts, Executive Office of Energy and Environmental Affairs. Uranium Activity to Mass Conversion Factor Guideline for Use in Drinking Water Compliance Monitoring and Risk Assessment. 2011. Available online: http://www.mass.gov/eea/agencies/massdep/water/regulations/uranium-activity-to-mass-conversion-factor-guideline.html (accessed on 11 December 2017).
- U.S. Environmental Protection Agency. 40 CFR Parts 9, 141, and 142; National Primary Drinking Water Regulations; Radionuclides, Final Rule. 7 December 2000. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/2000238H.PDF?Dockey=2000238H.PDF (accessed on 12 December 2017).
- Vogel, M.P. Household Drinking Water and Treatment; Montana State University Extension Service: Bozeman, MT, USA, 1991; 80p. [Google Scholar]
- Montana State University Extension Water Quality Program. Taking Care of Your Ground Water, a Homeowner’s Guide to Well and Septic Systems. 2009. Available online: http://waterquality.montana.edu/docs/WELL_EDUCATED/Well_and_Septic_DVD/Educational_Videos2.shtml (accessed on 14 July 2010).
- U.S. Environmental Protection Agency. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 13 December 2017).
- U.S. Environmental Protection Agency. Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. 2017. Available online: https://www.epa.gov/dwstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals (accessed on 13 December 2017).
- Szabo, Z. Invited keynote: Geochemistry as a critical factor in defining radionuclide occurrence in water from principal drinking-water aquifers of the United States. In Proceedings of the 5th International Conference on Medical Geology, Arlington, VA, USA, 25–29 August 2013; Volume 27. Available online: https://gsa.confex.com/gsa/2013MED/webprogram/Paper221628.html (accessed on 7 June 2017).
- Montana Natural Resources Information System (NRIS). State of Montana. Available online: http://nris.mt.gov (accessed on 15 November 2011).
- Wyoming Geographic Information Center. Available online: http://wygl.wygisc.org/wygeolib (accessed on 15 November 2011).
- Knows His Gun McCormick, A.; Pease, B.; Lefthand, M.J.; Eggers, M.J.; McCleary, T.; Felicia, D.; Camper, A.K. Water, a Resource for Health: Understanding impacts of water contamination in a Native American community. In Proceedings of the American Public Health Association Conference, San Francisco, CA, USA, 27–31 October 2012; Available online: https://apha.confex.com/apha/140am/webprogram/Paper261830.html (accessed on 1 April 2014).
- Swistock, B.R.; Clemens, S.; Sharpe, W.E. Drinking Water Quality in Rural Pennsylvania and the Effect of Management Practices; School of Forest Resources and Institutes of Energy and the Environment, Pennsylvania State University: State College, PA USA, 2009; 24p., Available online: http://www.rural.palegislature.us/drinking_water_quality.pdf (accessed on 3 March 2017).
- U.S. Department of Agriculture; U.S. Environmental Protection Agency. Private Drinking Water Wells. 2016. Available online: https://www.epa.gov/privatewells (accessed on 23 August 2016).
- U.S. Environmental Protection Agency. Drinking Water Advisory: Consumer Acceptability Advice and Health Effects Analysis on Sulfate; EPA 822-R-03-007; EPA: Washington, DC, USA, 2003. Available online: https://www.epa.gov/sites/production/files/2014-09/documents/support_cc1)sulfate_healtheffects.pdf (accessed on 24 August 2016).
- U.S. Environmental Protection Agency. A Brief Guide to Mold, Moisture, and Your Home; EPA: Washington, DC, USA, 2010. Available online: https://www.epa.gov/sites/production/files/2014-08/documents/moldguide.pdf (accessed on 25 August 2016).
- Malczewska-Toth, B.; Myers, O.; Lewis, J. Recommendations for a Uranium Health-Based Ground Water Standard; New Mexico Environment Department, Ground Water Quality Bureau: Santa Fe, NM, USA, 2003. [Google Scholar]
- Li, Z. Health risk characterization of maximum legal exposures for persistent organic pollutant (POP) pesticides in residential soil: An analysis. J. Environ. Manag. 2018, 205, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jennings, A. Worldwide regulations of standard values of pesticides for human health risk control: A review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Ander, E.L.; Watts, M.J.; Smedley, P.L.; Hamilton, E.M.; Close, R.; Crabbe, H.; Fletcher, T.; Rimell, A.; Studden, M.; Leonardi, G. Variability in the chemistry of private drinking water supplies and the impact of domestic treatment systems on water quality. Environ. Geochem. Health 2016, 38, 1313–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washington State Department of Agriculture; WA State Department of Ecology; WA State Department of Health; Yakima County Public Works Department; US Environmental Protection Agency. Lower Yakima Valley Groundwater Quality: Preliminary Assessment and Recommendations Document; Ecology Publication No. 10-10-009; WA State Department of Ecology: Lacey, WA, USA, 2010.
- Nolan, B.T.; Ruddy, B.C.; Hitt, K.J.; Helsel, D.R. A national look at nitrate contamination of ground water. Water Cond. Purif. 1998, 39, 76–79. [Google Scholar]
- U.S. Department of Agriculture; National Agricultural Statistics Service. 2007 Census of Agriculture. American Indian Reservations; Subject Series; Part 5AC-07-S-5; 2007; Volume 2. Available online: http://www.agcensus.usda.gov/PUublications/2007/Online_Highlights/American_Indian_Reservations/amind_09.pdf (accessed on 20 April 2014).
- Moore-Nall, A. The legacy of uranium development on or near Indian Reservations and health implications rekindling public awareness. Geosciences 2015, 5, 15–29. [Google Scholar] [CrossRef]
- Nolan, J.; Weber, K.A. Natural uranium contamination in major U.S. aquifers linked to nitrate. Environ. Sci. Technol. Lett. 2015, 2, 215–220. [Google Scholar] [CrossRef]
- Schnug, E.; Lottermoser, B.G. Fertilizer-derived uranium and its threat to human health. Environ. Sci. Technol. 2013, 47, 2433–2434. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Smith, J.; Naidu, R. Distribution and nature of arsenic along former railway corridors of South Australia. Sci. Total Environ. 2005, 363, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Massachusetts Department of Environmental Protection, Bureau of Waste Site Cleanup. Best Management Practices for Controlling Exposure to Soil during the Development of Rail Trails. Available online: http://webcache.googleusercontent.com/search?q=cache, (accessed on 22 January 2017).
- Mark, D.; (Bighorn Valley Health Center, Hardin, MT, USA); Byron, R.; (Bighorn Valley Health Center, Hardin, MT, USA). Personal communication, 2015.
- Shan, Z.; Chen, S.; Sun, T.; Luo, C.; Guo, Y.; Yu, X.; Yang, W.; Hu, F.B.; Liu, L. U-Shaped association between plasma manganese levels and type 2 diabetes. Environ. Health Perspect. 2016, 124, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Feng, W.; Wang, J.; Li, Y.; Han, X.; Hu, H.; Guo, H.; Zhang, X. Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ. Pollut. 2016, 210, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Tondel, M.; Ahmad, S.A.; Axelson, O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am. J. Epidemiol. 1998, 148, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Tai, T.-Y.; Chong, C.-K.; Tseng, C.-P.; Lai, M.-S.; Lin, B.J.; Chiou, H.-Y.; Hsueh, Y.-M.; Hsu, K.-H.; Chen, C.-J. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: A cohort study in arseniasis-hyperendemic villages in Taiwan. Environ. Health Perspect. 2000, 108, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Sexton, K.; Hattis, D. Assessing cumulative health risks from exposure to environmental mixtures—Three fundamental questions. Environ. Health Perspect. 2007, 115, 825–832. [Google Scholar] [CrossRef] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Strontium; ATSDR: Atlanta, GA, USA, 2004. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp159.pdf (accessed on 28 May 2017).
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Boron; ATSDR: Atlanta, GA, USA, 2010. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp26.pdf (accessed on 28 May 2017).
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine; ATSDR: Atlanta, GA, USA, 2012. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp11.pdf (accessed on 28 May 2017).
- Pelizza, M. Uranium and uranium progeny in groundwater associated with uranium ore bearing formations. In Proceedings of the 5th International Conference on Medical Geology, Arlington, VA, USA, 25–29 August 2013; Volume 27. Available online: https://gsa.confex.com/gsa/2013MED/webprogram/Paper220877.html (accessed on 7 June 2017).
- Caldwell, R. Technical Announcement. USGS Samples for Radioactive Constituents in Groundwater of Southwestern Montana. 2011. Available online: http://mt.water.usgs.gov/ (accessed on 7 August 2013).
- Li, Z.; Jennings, A.A. Implied maximum dose analysis of standard values of 25 pesticides based on major human exposure pathways. AIM Public Health 2017, 4, 383–398. [Google Scholar] [CrossRef]
- Barbeau, B.; Carriere, A.; Bouchard, M.F. Spatial and temporal variations of manganese concentrations in drinking water. J. Environ. Sci. Health A Toxin Hazard. Subst. Environ. Eng. 2015, 46, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Sigler, W.A.; Ewing, S.A.; Jones, C.A.; Payn, R.A.; Brookshire, E.N.J.; Klassen, J.K.; Jackson-Smith, D.; Weissmann, G.S. Connections among soil, ground, and surface water chemistries characterize nitrogen loss from an agricultural landscape in the upper Missouri River basin. J. Hydrol. 2017. [Google Scholar] [CrossRef]
- Cheng, Z.; Van Geen, A.; Seddique, A.A.; Ahmed, K.M. Limited temporal variability of arsenic concentrations in 20 wells monitored for 3 years in Araihazar, Bangladesh. Environ. Sci. Technol. 2005, 39, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Probabilistic Risk Analysis Technical Panel; Risk Assessment Forum, Office of the Science Advisor; U.S. Environmental Protection Agency. Risk Assessment Forum White Paper: Probabilistic Risk Assessment Methods and Case Studies. 2014. Available online: https//www.epa.gov/osa/probabilistic-risk-assessment-white-papre-and-supporting-documents (accessed on 26 December 2017).
- National Health and Nutrition Examination Survey, Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 4 November 2017).
- Behavioral Risk Factor Surveillance System, Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/brfss/index.html (accessed on 4 November 2017).
- International Human Rights Law Clinic, University of California Berkeley, School of Law. The Human Right to Water Bill in California: An Implementation Framework for State Agencies; University of California Berkeley: Berkeley, CA, USA, 2013; Available online: www.ushrnetwork.org/sites/ushrnetwork.org/files/ihrlc_water_report_2013_final.pdf (accessed on 31 December 2017).


| Water Quality Parameter | Method a | Reporting Limit | Unit |
|---|---|---|---|
| Metals | |||
| Aluminum | E200.8 b | 0.1 | mg/L |
| Arsenic | E200.8 | 0.001 | mg/L |
| Cadmium | E200.8 | 0.0001 | mg/L |
| Calcium | E200.7 c | 1 | mg/L |
| Chromium | E200.8 | 0.001 | mg/L |
| Iron | E200.7 | 0.03 | mg/L |
| Lead | E200.8 | 0.001 | mg/L |
| Magnesium | E200.7 | 1 | mg/L |
| Manganese | E200.7/E200.8 d | 0.01 | mg/L |
| Potassium | E200.7 | 1 | mg/L |
| Sodium | E200.7 | 1 | mg/L |
| Uranium | E200.8 | 0.001 | mg/L |
| Zinc | E200.7/E200.8 | 0.01 | mg/L |
| Inorganics | |||
| Alkalinity | A2320 B | 4 | mg/L |
| Bicarbonate | A2320 B | 4 | mg/L |
| Carbonate | A2320 B | 4 | mg/L |
| Chloride | E300.0 | 1 | mg/L |
| Sulfate | E300.0 | 1 | mg/L |
| Fluoride | E300.0 | 0.1 | mg/L |
| Nitrate + Nitrite as N | E300.0 | 0.05 | mg/L |
| Hardness as CaCO3 | A2340 B | 1 | mg/L |
| Sodium Absorption Ratio | Calculation | 0.01 | |
| Physical Properties | |||
| Conductivity | A2510 B | 1 | µmhos/cm |
| Corrosivity (Langelier Index) | A203 | ||
| pH | A4500 H B | 0.1 | s.u. |
| Total Dissolved Solids @ 180 C | A2540 C | 10 | mg/L |
| Coliform/E. coli presence/absence | A9223 | presence/absence | per 100 mL |
| Contaminant | Oral RfD (mg/kg-day) | Citation |
|---|---|---|
| Arsenic | 3 × 10−4 | [134] |
| Cadmium | 5.0 × 10−4 | [134] |
| Chromium | 3.0 × 10−3 | [134] |
| Manganese | 4.6 × 10−2 | [134,135,136] |
| Nitrate | 1.6 | [134] |
| Uranium | 2 × 10−4 | [137] |
| Zinc | 0.3 | [134] |
| Analyte | n | Min (mg/L) | Avg conc (mg/L) ± SD (mg/L) | Max (mg/L) | Number of Detections | Percent Detection |
|---|---|---|---|---|---|---|
| Nitrate + Nitrite as N | 164 | <0.05 | 1.61 ± 5.13 | 39.8 | 70 | 42.7 |
| As | 164 | <0.001 | 0.0012 ± 0.0025 | 0.011 | 45 | 27.4 |
| Mn | 164 | <0.01 | 0.102 ± 0.215 | 1.35 | 85 | 51.8 |
| Zn | 164 | <0.01 | 0.11 ± 0.01 | 9.15 | 80 | 48.8 |
| U | 97 | <0.001 | 0.008 ± 0.014 | 0.101 | 66 | 68.0 |
| Cd | 55 | <0.001 | N/A | N/A | 0 | 0 |
| Cr | 55 | <0.01 | N/A | 0.01 | 4 | 7.3 |
| TDS | 164 | 238 | 1425 ± 1215 | 9180 | 164 | 100.0 |
| Sulfate | 164 | <1 | 682 ± 765 | 4750 | 163 | 99.4 |
| Iron | 155 | <0.05 | 0.71 ± 2.19 | 21.7 | 79 | 51.0 |
| Contaminant | EPA Standard [143] | Percent of Crow Wells in Use Exceeding EPA Standards a n = 164; n for U = 97; n for Fe = 155 | Percent of Crow Wells When Drilled Exceeding EPA Standards b n = 470–~650 | Percent of United States Wells Exceeding EPA Standards c n = 1725–2160 |
|---|---|---|---|---|
| Primary Standard | ||||
| Mn ≥ 0.30 mg/L | HA | 11.0 | 17.0 | 5.2 |
| As > 0 mg/L | MCLG | 27.4 | ||
| As ≥ 0.01 mg/L | MCL | 1.2 | 6.8 | |
| U > 0 mg/L | MCLG | 68.0 | ||
| U ≥ 0.030 mg/L | MCL | 6.2 | 1.7 | |
| NO3− ≥ 10.0 mg/L | MCL | 4.3 | 5.0 | 4.4 |
| Coliform present | MCL | 40.2 | ||
| E. coli present | MCL | 0.6 | ||
| Secondary standard | ||||
| TDS > 500 mg/L | SMCL | 85.4 | 93.0 | 14.8 |
| SO42− > 250 mg/L | SMCL | 68.9 | 75.0 | 3.8 |
| Mn > 0.05 mg/L | SMCL | 32.9 | 57.5 | 21.3 |
| Fe > 0.3 mg/L | SMCL | 25.0 | 63.0 | 19.1 |
| Hardness > 120 mg/L | SMCL | 76.8 | 62.0 | |
| River Valley, from West to East on the Crow Reservation | Number of Home Wells | Wells with Sum of RQs > 1.0: Percent ± SE | Average Sum of RQs ± SE |
|---|---|---|---|
| Pryor Creek | 9 | 11 ± 11% | 0.51 ± 0.14 |
| Bighorn River Valley | 14 | 64 ± 13% | 2.16 ± 0.64 |
| Little Bighorn River Valley | 74 | 17 ± 4% | 0.73 ± 0.03 |
| Crow Agency | 13 | 23 ± 12% | 1.09 ± 0.37 |
| Garryowen | 12 | 8 ± 8% | 0.57 ± 0.18 |
| Lodge Grass | 24 | 8 ± 5% | 0.52 ± 0.18 |
| Wyola | 25 | 28 ± 9% | 0.84 ± 0.19 |
| Total Number of Wells | 97 | ||
| Reservation Wide Average | 24 ± 4% | 0.92 ± 0.03 |
| Predictor(s) | Dependent Variable | n | R2 | Regression Significance | Regression Equation |
|---|---|---|---|---|---|
| logTDS | log[Mn] | 151 | 0.164 | p < 0.0005 | log[Mn] = −4.60 + 0.961 logTDS |
| log[Fe] | log[Mn] | 163 | 0.419 | p < 0.0005 | log[Mn] = −1.08 + 0.626 log[Fe] |
| logTDS, log[Fe] | log[Mn] | 151 | 0.503 | log TDS: p < 0.0005; log[Fe]: p < 0.0005 | log[Mn] = −3.27 + 0.707 logTDS + 0.574 log[Fe] |
| logTDS | log[U] | 97 | 0.194 | p < 0.0005 | log[U] = −5.463 + 0.9525 logTDS |
| pH | log[U] | 97 | 0.341 | p < 0.0005 | log[U] = 3.45 − 0.795 pH |
| log[NO3−] | log[U] | 97 | 0.160 | p < 0.0005 | log[U] = −2.34 + 0.267 log[NO3−] |
| logTDS, pH, log[NO3−] | log[U] | 97 | 0.579 | log TDS: p < 0.0005; pH: p < 0.0005; log[NO3−]: 0.001 | log[U] = −0.016 + 0.905 logTDS − 0.686 pH + 0.167 log[NO3−] |
| Well Water Contaminant | n | Avg ADD * (mg/L) | RfD (mg/L) | # HQ ≥ 1.0 | % HQ ≥ 1.0 | Avg HQ | MCL (mg/L) | # > MCL | % > MCL | Avg RQ * |
|---|---|---|---|---|---|---|---|---|---|---|
| Nitrate + Nitrite as N | 164 | 0.05 | 1.6 | 0 | 0 | 0.03 | 10.0 | 7 | 4.3% | 0.16 |
| As | 164 | 3 × 10−5 | 0.0003 | 2 | 1.2% | 0.11 | 0.01 | 2 | 1.2% | 0.12 |
| Mn | 164 | 0.03 | 0.046 | 0 | 0 | 0.06 | 0.3 | 18 | 11.0% | 0.34 |
| Zn | 164 | 0.003 | 0.3 | 0 | 0 | 0.01 | N/A | N/A | N/A | N/A |
| U | 97 | 0.0002 | 0.0002 | 32 | 33.0% | 1.16 | 0.03 | 6 | 6.30% | 0.27 |
| Hazard Indices (Sum of Hazard Quotients) Based on Oral Reference Doses (RfDs) | Sum of Risk Quotients on EPA Water Quality Maximum Contaminant Levels (MCLs) | ||
|---|---|---|---|
| Number of wells with HI > 1.0 | 38 | Number of wells with summed RQ > 1.0 | 23 |
| Percent of wells with HI > 1.0 | 39.2 | Percent of wells with summed RQ > 1.0 | 23.7 |
| Average HI of wells | 1.40 | Average summed RQs of wells | 0.93 |
| Carcinogenic Risk from Arsenic | Carcinogenic Risk from Uranium | ||
|---|---|---|---|
| Number of wells tested for arsenic, reporting limit of 0.001 mg/L | 164 | Number of wells tested for uranium, reporting limit of 0.001 mg/L | 97 |
| Number of wells with detected arsenic | 44 | Number of wells with detected uranium | 66 |
| Percent of wells with detected arsenic | 26.8 | Percent of wells with detected uranium | 68.8 |
| Number of wells with carcinogenic risk ≥ 4 × 10−5 | 44 | Number of wells with carcinogenic risk ≥ 1.0 × 10−6 | 0 |
| Percent of wells with carcinogenic risk ≥ 4 × 10−5 | 26.8 | Percent of wells with carcinogenic risk ≥ 1.0 × 10−6 | 0.0 |
| Families’ Well Water Use | n (%) | TDS (mg/L) Mean ± SD | Wells Assessed for HI | |||
|---|---|---|---|---|---|---|
| n | Number with HI ≥ 1.0 | Percent with HI > 1.0 | Mean HI ± SD | |||
| Drink & cook | 91 (59.9%) | 959 ± 578 | 55 | 14 | 26.4 | 0.7 ± 0.8 |
| Cook, only | 31 (20.4%) | 1970 ± 1466 | 21 | 9 | 42.8 | 2.1 ± 3.6 |
| Do not consume | 30 (19.7%) | 2262 ± 1726 | 23 | 15 | 65.2 | 2.4 ± 2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggers, M.J.; Doyle, J.T.; Lefthand, M.J.; Young, S.L.; Moore-Nall, A.L.; Kindness, L.; Other Medicine, R.; Ford, T.E.; Dietrich, E.; Parker, A.E.; et al. Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana. Int. J. Environ. Res. Public Health 2018, 15, 76. https://doi.org/10.3390/ijerph15010076
Eggers MJ, Doyle JT, Lefthand MJ, Young SL, Moore-Nall AL, Kindness L, Other Medicine R, Ford TE, Dietrich E, Parker AE, et al. Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana. International Journal of Environmental Research and Public Health. 2018; 15(1):76. https://doi.org/10.3390/ijerph15010076
Chicago/Turabian StyleEggers, Margaret J., John T. Doyle, Myra J. Lefthand, Sara L. Young, Anita L. Moore-Nall, Larry Kindness, Roberta Other Medicine, Timothy E. Ford, Eric Dietrich, Albert E. Parker, and et al. 2018. "Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana" International Journal of Environmental Research and Public Health 15, no. 1: 76. https://doi.org/10.3390/ijerph15010076
APA StyleEggers, M. J., Doyle, J. T., Lefthand, M. J., Young, S. L., Moore-Nall, A. L., Kindness, L., Other Medicine, R., Ford, T. E., Dietrich, E., Parker, A. E., Hoover, J. H., & Camper, A. K. (2018). Community Engaged Cumulative Risk Assessment of Exposure to Inorganic Well Water Contaminants, Crow Reservation, Montana. International Journal of Environmental Research and Public Health, 15(1), 76. https://doi.org/10.3390/ijerph15010076