Antibiotics in Crab Ponds of Lake Guchenghu Basin, China: Occurrence, Temporal Variations, and Ecological Risks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Ponds and Sample Collection
2.2. Chemicals and Standards
2.3. Sample Preparation and Analysis
2.4. Quality Assurance and Quality Control
2.5. Risk Characterization
2.6. Environmental Parameters Determination and Statistical Analysis
3. Results
3.1. Antibiotics in Crab Ponds of Lake Guchenghu Basin
3.2. Relationship between Antibiotics and Environmental Parameters
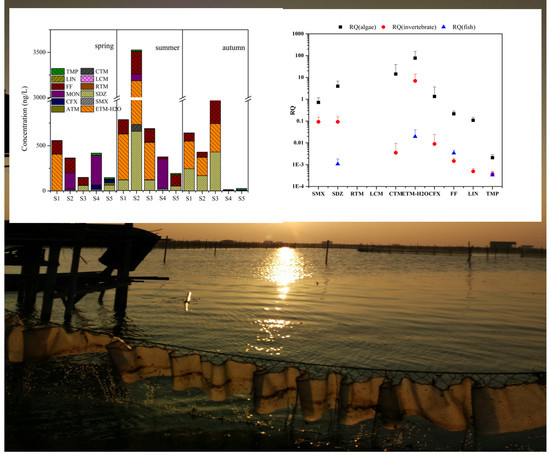
3.3. Risk Assessment
4. Discussion
4.1. Pollution Levels of Antibiotics in Crab Ponds of Lake Guchenghu Basin
4.2. Temporal Variations of Antibiotics and Correlative Environmental Factors in Crab Ponds
4.3. Environmental Application
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Archundia, D.; Duwig, C.; Lehembre, F.; Chiron, S.; Morel, M.C.; Prado, B.; Bourdat-Deschamps, M.; Vince, E.; Flores Aviles, G.; Martins, J.M.F. Antibiotic pollution in the Katari subcatchment of the Titicaca Lake: Major transformation products and occurrence of resistance genes. Sci. Total Environ. 2017, 576, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B.; Khan, G.A.; Lindberg, R.; Fick, J.; Lindgren, P.E. Abundance and Dynamics of Antibiotic Resistance Genes and Integrons in Lake Sediment Microcosms. PLoS ONE 2014, 9, e108151. [Google Scholar] [CrossRef] [PubMed]
- Hladicz, A.; Kittinger, C.; Zarfel, G. Tigecycline Resistant Klebsiellapneumoniae Isolated from Austrian River Water. Int. J. Environ. Res. Public Health 2017, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Bound, J.P.; Voulvoulis, N. Pharmaceuticals in the Aquatic Environment––A Comparison of Risk Assessment Strategies. Chemosphere 2004, 55, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.Z.; Jin, L.; Jiang, L.; Han, Q.; Lin, K.F.; Lu, S.G.; Zhang, D.; Cao, G.M. Removal of Trace Level Amounts of Twelve Sulfonamides from Drinking Water by UV-activated Peroxymonosulfate. Sci. Total Environ. 2016, 572, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Takada, H.; Mutoh, K.; Hosoda, H.; Harada, A.; Nakada, N. Nationwide Monitoring of Selected Antibiotics: Distribution and Sources of Sulfonamides, Trimethoprim, and Macrolides in Japanese Rivers. Sci. Total Environ. 2011, 409, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Ying, G.G.; Zhao, J.L.; Tao, R.; Su, H.C.; Chen, F. Simultaneous Determination of Four Classes of Antibiotics in Sediments of the Pearl Rivers Using RRLC–MS/MS. Sci. Total Environ. 2010, 408, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Gibs, J.; Heckathorn, H.A.; Meyer, M.T.; Klapinski, F.R.; Alebus, M.; Lippincott, R.L. Occurrence and Partitioning of Antibiotic Compounds Found in the Water Column and Bottom Sediments from a Stream Receiving Two Wastewater Treatment Plant Effluents in Northern New Jersey, 2008. Sci. Total Environ. 2013, 458–460, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Wu, Q.L.; Zhang, B.B.; Zhao, Y.G.; Zhao, B.Y. Occurrence, Spatiotemporal Distribution, Mass Balance and Ecological Risks of Antibiotics in Subtropical Shallow Lake Taihu, China. Environ. Sci. Process. Impacts 2016, 18, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, Y.; Tong, L.; Deng, Y.; Li, Y.; Gan, Y.; Guo, W.; Dong, C.; Duan, Y.; Zhao, K. Occurrence and Risk Assessment of Antibiotics in Surface Water and Ground Water from Different Depths of Aquifers: A Case Study at Jianghan Plain, Central China. Ecotoxicol. Environ. Saf. 2017, 135, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xu, X.R.; Zhou, G.J.; Liu, S.S.; Yue, W.Z. Antibiotics in the Coastal Environment of the Hailing Bay Region, South China Sea: Spatial Distribution, Source Analysis and Ecological Risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Carlson, K. Temporal and Spatial Trends in the Occurrence of Human and Veterinary Antibiotics in Aqueous and River Sediment matrices. Environ. Sci. Technol. 2007, 41, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.F.; Ye, Y.T.; Chen, L.Q.; Qin, J.G.; Wang, Y.L. Oxygen consumption and ammonia excretion of black carp (Mylopharyngdon piceus Richardson) and allogynogenetic crucian carp (Carassius auratus gibelio ♀ × Cyprinus carpio ♂) fed different carbohydrate diets. Fish Physiol. Biochem. 2010, 36, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, C.; Fan, L.M.; Qiu, L.P.; Wu, W.; Meng, S.L.; Hu, G.D.; Kamira, B.; Chen, J.Z. Occurrence of Antibiotics and Their Impacts to Primary Productivity in Fishponds around Tai Lake, China. Chemosphere 2016, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, F.; Meng, F.G.; Xie, Y.Y.; Chen, H.; Young, K.; Luo, W.X.; Ye, T.J.; Fu, W.J. Occurrence and Fate of PPCPs and Correlations with Water Quality Parameters in Urban Riverine Waters of the Pearl River Delta, South China. Environ. Sci. Pollut. Res. 2013, 20, 5864–5875. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.F.; Gu, X.H.; Chen, X.; Mao, Z.G. The Impact of Chinese Mitten Crab Culture on Water Quality, Sediment and the Pelagic and Macrobenthic Community in the Reclamation Area of Guchenghu Lake. Fish Sci. 2013, 79, 689–697. [Google Scholar] [CrossRef]
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhao, J.L.; Chen, F.; Zhang, R.Q.; Peng, F.Q.; Zhang, Q.Q. Simultaneous Determination of Human and Veterinary Antibiotics in Various Environmental Matrices by Rapid Resolution Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A. 2012, 1244, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Shi, Y.L.; Gao, L.H.; Liu, J.M.; Cai, Y.Q. Occurrence of Antibiotics in Water, Sediments, Aquatic Plants, and Animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the Surface Water of the Yangtze Estuary: Occurrence, Distribution and Risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21th ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- LepŠ, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Xuan, L.T.; Munekage, Y. Residues of Selected Antibiotics in Water and Mud from Shrimp Ponds in Mangrove Areas in VietNam. Mar. Pollut. Bull. 2004, 49, 922–929. [Google Scholar]
- Romero-González, R.; López-Martínez, J.C.; Gómez-Milán, E.; Garrido-Frenich, A.; Martínez-Vidal, J.L. Simultaneous Determination of Selected Veterinary Antibiotics in Gilthead Seabream (sparusaurata) by Liquid Chromatography–Mass Spectrometry. J. Chromatogr. B. 2007, 857, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Na, G.S.; Fang, X.D.; Cai, Y.Q.; Ge, L.K.; Zong, H.M.; Yuan, X.T.; Yao, Z.W.; Zhang, Z.F. Occurrence, Distribution, and Bioaccumulation of Antibiotics in Coastal Environment of Dalian, China. Mar. Pollut. Bull. 2013, 69, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and Transport of Tetracycline, Sulfonamide, Quinolone, and Macrolide Antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, J.; Li, J.; Cheng, Z.; Chaemfa, C.; Liu, D.; Zheng, Q.; Song, M.; Luo, C.; Zhang, G. Occurrence and Risks of Antibiotics in the Coastal Aquatic Environment of the Yellow Sea, North China. Sci. Total Environ. 2013, 450, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analysis of macrolide antibiotics, using liquid chromatography-mass spectrometry, in food, biological and environmental matrices. Mass Spectrom. Rev. 2009, 28, 50–92. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shi, T.Z.; Wu, X.W.; Cao, H.Q.; Li, X.D.; Hua, R.M.; Tang, F.; Yue, Y.D. The Occurrence and Distribution of Antibiotics in Lake Chaohu, China: Seasonal Variation, Potential Source and Risk Assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hoa, P.T.; Managaki, S.; Nakada, N.; Takada, H.; Shimizu, A.; Anh, D.H.; Viet, P.H.; Suzuki, S. Antibiotic Contamination and Occurrence of Antibiotic-resistant Bacteria in Aquatic Environments of Northern Vietnam. Sci. Total Environ. 2011, 409, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.W.; Meng, W.; Xu, J.; Zhang, Y.; Guo, C.S. Occurrence, Distribution and Bioaccumulation of Antibiotics in the Liao River Basin in China. Environ. Sci. Process. Impacts 2014, 16, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.L. Occurrence of Antibiotics in the Aquatic Environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhou, C.B.; Guo, C.S.; Wang, D.M.; Du, P.; Luo, Y.; Wan, J.; Meng, W. Distribution, Sources and Composition of Antibiotics in Sediment, Overlying Water and Pore Water from Taihu Lake, China. Sci. Total Environ. 2014, 497, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Andreu, V. Fluoroquinolones in Soil-Risks and Challenges. Anal. Bioanal. Chem. 2007, 387, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Matyar, F.; Gulnaz, O.; Guzeldag, G.; Mercimek, H.A.; Akturk, S.; Arkut, A.; Sumengen, M. Antibiotic and Heavy Metal Resistance in Gram-negative Bacteria Isolated from the Seyhan Dam Lake and Seyhan River in Turkey. Ann. Microbiol. 2014, 64, 1033–1040. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and other Organic Waste Contaminants in U.S. streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of Antibiotics in Wastewater Treatment Facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef] [PubMed]
- He, B.Y.; Dai, M.H.; Zhai, W.D.; Wang, L.F.; Wang, K.J.; Chen, J.H.; Lin, J.R.; Han, A.Q.; Xu, Y.P. Distribution, Degradation and Dynamics of Dissolved Organic Carbon and Its Major Compound Classes in the Pearl River Estuary, China. Mar. Chem. 2010, 119, 52–64. [Google Scholar] [CrossRef]
- Wu, T.F.; Qin, B.Q.; Zhu, G.W.; Luo, L.C.; Ding, Y.Q.; Bian, G.Y. Dynamics of Cyanobacterial Bloom Formation during Short-term Hydrodynamic Fluctuation in a Large Shallow, Eutrophic, and Wind-exposed Lake Taihu, China. Environ. Sci. Pollut. Res. 2013, 20, 8546–8556. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling Harmful Cyanobacterial Blooms in a World Experiencing Anthropogenic and Climatic-induced Change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dolz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial Use in Aquaculture Re-examined: Its Relevance to Antimicrobial Resistance and to Animal and Human Health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jia, A.; Wan, Y.; Liu, H.; Wang, K.; Peng, H.; Dong, Z.; Hu, J. Occurrences of Three Classes of Antibiotics in a Natural River Basin: Association with Antibiotic-resistant Escherichia coli. Environ. Sci. Technol. 2014, 48, 14317–14325. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Gu, X.; Zhou, L.; Chen, H.; Zeng, Q.; Mao, Z. Antibiotics in Crab Ponds of Lake Guchenghu Basin, China: Occurrence, Temporal Variations, and Ecological Risks. Int. J. Environ. Res. Public Health 2018, 15, 548. https://doi.org/10.3390/ijerph15030548
Wang W, Gu X, Zhou L, Chen H, Zeng Q, Mao Z. Antibiotics in Crab Ponds of Lake Guchenghu Basin, China: Occurrence, Temporal Variations, and Ecological Risks. International Journal of Environmental Research and Public Health. 2018; 15(3):548. https://doi.org/10.3390/ijerph15030548
Chicago/Turabian StyleWang, Wenxia, Xiaohong Gu, Lijun Zhou, Huihui Chen, Qingfei Zeng, and Zhigang Mao. 2018. "Antibiotics in Crab Ponds of Lake Guchenghu Basin, China: Occurrence, Temporal Variations, and Ecological Risks" International Journal of Environmental Research and Public Health 15, no. 3: 548. https://doi.org/10.3390/ijerph15030548
APA StyleWang, W., Gu, X., Zhou, L., Chen, H., Zeng, Q., & Mao, Z. (2018). Antibiotics in Crab Ponds of Lake Guchenghu Basin, China: Occurrence, Temporal Variations, and Ecological Risks. International Journal of Environmental Research and Public Health, 15(3), 548. https://doi.org/10.3390/ijerph15030548