Occurrence and Risk Assessment of PAHs in Surface Sediments from Western Arctic and Subarctic Oceans
Abstract
:1. Introduction
2. Sampling Strategy and Methods
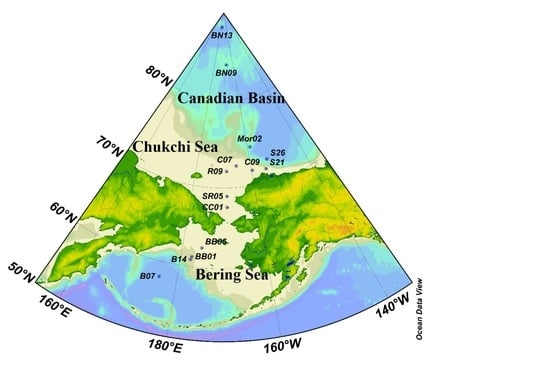
2.1. Study Area and Sample Collection
2.2. Analysis of PAHs
2.2.1. Pre-Treatment of Sediments, PAHs Extraction, and Cleanup
2.2.2. Instrumental Analysis of PAHs
2.2.3. Quality Assurance and Quality Control
3. Results and Discussion
3.1. Sediment Properties (Grain Size)
3.2. Concentration and Composition
3.2.1. PAHs Levels and Compositions
3.2.2. Comparison with Other Places in Arctic Ocean
3.3. PAHs Spatial Distribution
3.4. Potential Source of PAHs
3.5. Ecological Risk and Health Risk Assessment
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, X.; Wang, X.-M.; Xie, Z.-Q.; Xiang, C.-H.; Mai, B.-X.; Sun, L.-G.; Zheng, M.; Sheng, G.-Y.; Fu, J.-M.; Pöschl, U. Atmospheric polycyclic aromatic hydrocarbons observed over the North Pacific Ocean and the Arctic area: Spatial distribution and source identification. Atmos. Environ. 2007, 41, 2061–2072. [Google Scholar] [CrossRef]
- Grung, M.; Petersen, K.; Fjeld, E.; Allan, I.; Christensen, J.H.; Malmqvist, L.M.V.; Meland, S.; Ranneklev, S. PAH related effects on fish in sedimentation ponds for road runoff and potential transfer of PAHs from sediment to biota. Sci. Total Environ. 2016, 566, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.L.; Gu, Y.G.; Liu, Q.; Li, L.D.; Huang, H.H.; Cai, N.; Sun, Z.W. Polycyclic aromatic hydrocarbons (PAHs) in wild marine organisms from South China Sea: Occurrence, sources, and human health implications. Mar. Pollut. Bull. 2017, 117, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, S.M.; Singleton, I. Bioremediation of polycyclic aromatic hydrocarbons: Current knowledge and future directions. J. Chem. Technol. Biotechnol. 2005, 80, 723–736. [Google Scholar] [CrossRef]
- Duran, R.; Cravolaureau, C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol. Rev. 2016, 40, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liu, M.; Hong, Q.; Lin, J.; Huang, P.; Hong, J.; Wang, J.; Zhao, W.; Chen, M.; Cai, M. Fate of PAHs in Seawater from the Western Pacific to the Southern Ocean (17.5°N–69.2°S) and their Inventories on the Antarctic Shelf. Environ. Sci. Technol. 2016, 24, 9161–9168. [Google Scholar] [CrossRef] [PubMed]
- Niimi, A.J.; Palazzo, V. Biological half-lives of eight polycyclic aromatic hydrocarbons (PAHs) in rainbow trout (Salmo gairdneri). Water Res. 1986, 20, 503–507. [Google Scholar] [CrossRef]
- Yim, U.H.; Hong, S.H.; Shim, W.J. Distribution and characteristics of PAHs in sediments from the marine environment of korea. Chemosphere 2007, 68, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.M.; Stout, S.A.; Gunster, D.G. Ecological risk assessment of polycyclic aromatic hydrocarbons in sediments: Identifying sources and ecological hazard. Integr. Environ. Assess. Manag. 2005, 1, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kilemade, M.; Hartl, M.G.; Sheehan, D.; Mothersill, C.; van Pelt, F.N.; O’Brien, N.M.; O’Halloran, J. An assessment of the pollutant status of surficial sediment in Cork Harbour in the South East of Ireland with particular reference to polycyclic aromatic hydrocarbons. Mar. Pollut. Bull. 2004, 49, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Notar, M.; Leskovsek, H.; Faganeli, J. Composition, distribution and sources of polycyclic aromatic hydrocarbons in sediments of the Gulf of Trieste, Northern Adriatic Sea. Mar. Pollut. Bull. 2001, 42, 36–44. [Google Scholar] [CrossRef]
- Ma, Y.X.; Halsall, C.J.; Crosse, J.D.; Graf, C.; Cai, M.H.; He, J.F.; Gao, G.P.; Jones, K. Persistent organic pollutants in ocean sediments from the North Pacific to the Arctic Ocean. J. Geophys. Res. Oceans 2015, 120, 2723–2735. [Google Scholar] [CrossRef] [Green Version]
- Araghi, P.E.; Bastami, K.D.; Rahmanpoor, S. Distribution and sources of polycyclic aromatic hydrocarbons in the surface sediments of Gorgan Bay, Caspian Sea. Mar. Pollut. Bull. 2014, 89, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Jimenez-Guerrero, P.; Ratola, N. Levels, trends and health concerns of atmospheric PAHs in Europe. Atmos. Environ. 2014, 99, 474–484. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X. Polycyclic aromatic hydrocarbons in sediments of China Sea. Environ. Sci. Pollut. Res. 2015, 22, 15432–15442. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Halsall, C.J.; Xie, Z.; Koetke, D.; Mi, W.; Ebinghaus, R.; Gao, G. Polycyclic aromatic hydrocarbons in ocean sediments from the North Pacific to the Arctic Ocean. Environ. Pollut. 2017, 227, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; Abedi, E.; Mohammady, M.; Faghiri, I.; Fakhri, R.; Azimi, A. Distribution and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Sediments from the Northern Part of the Persian Gulf (Hormuzgan Province). Polycycl. Aromat. Compd. 2014, 34, 343–355. [Google Scholar] [CrossRef]
- Nouira, T.; Tagorti, M.A.; Budzinski, H.; Etchebert, H.; Boussetta, H. Polycyclic aromatic hydrocarbons (PAHs) in surface sediments of Monastir Bay (Tunisia, Central Mediterranean): Distribution, origin and seasonal variations. Int. J. Environ. Anal. Chem. 2013, 93, 1470–1483. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Xu, K.; Mayer, L.M.; Zhang, Z.; Kolker, A.S.; Wu, W. Concentrations and sources of polycyclic aromatic hydrocarbons in surface coastal sediments of the northern Gulf of Mexico. Geochem. Trans. 2014, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.C.; De, V.P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Palm, A.; Cousins, I.; Gustafsson, O.; Axelman, J.; Grunder, K.; Broman, D.; Brorström-Lundén, E. Evaluation of sequentially-coupled POP fluxes estimated from simultaneous measurements in multiple compartments of an air-water-sediment system. Environ. Pollut. 2004, 128, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Mackay, D.; Jones, K.C.; Harner, T.; Meijer, S.N. Evidence for the “grasshopper” effect and fractionation during long-range atmospheric transport of organic contaminants. Environ. Pollut. 2004, 128, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Shu, T.; Wang, B.; Yu, Y.; Chang, L.; Zhang, Y.X.; Jing, H.; Ma, J.M.; Hung, H. Sources and pathways of polycyclic aromatic hydrocarbons transported to alert, the Canadian High Arctic. Environ. Sci. Technol. 2010, 44, 1017–1022. [Google Scholar]
- Sofowote, U.M.; Hung, H.; Rastogi, A.K.; Westgate, J.N.; Deluca, P.F.; Su, Y.; Mccarry, B.E. Assessing the long-range transport of pah to a sub-arctic site using positive matrix factorization and potential source contribution function. Atmos. Environ. 2011, 45, 967–976. [Google Scholar] [CrossRef]
- Weber, J.; Halsall, C.J.; Muir, D.C.; Teixeira, C.; Burniston, D.A.; Strachan, W.M.; Hung, H.; Mackay, N.; Arnold, D.; Kylin, H. Endosulfan and gamma-HCH in the arctic: An assessment of surface seawater concentrations and air-sea exchange. Environ. Sci. Technol. 2006, 40, 7570–7576. [Google Scholar] [CrossRef] [PubMed]
- Belicka, L.L.; Harvey, H.R. The sequestration of terrestrial organic carbon in Arctic Ocean sediments: A comparison of methods and implications for regional carbon budgets. Geochim. Cosmochim. Acta 2009, 73, 6231–6248. [Google Scholar] [CrossRef]
- Lohmann, R.; Gioia, R.; Jones, K.C.; Nizzetto, L.; Temme, C.; Xie, Z.; Schulz-Bull, D.; Hand, I.; Morgan, E.; Jantunen, L. Organochlorine Pesticides and PAHs in the Surface Water and Atmosphere of the North Atlantic and Arctic Ocean. Environ. Sci. Technol. 2009, 43, 5633–5639. [Google Scholar] [CrossRef] [PubMed]
- Bergamasco, A.; Culotta, L.; De Stefano, C.; Orecchio, S.; Sammartano, S.; Barreca, S. Composition, Distribution, and Sources of Polycyclic Aromatic Hydrocarbons in Sediments of the Gulf of Milazzo (Mediterranean Sea, Italy). Polycycl. Aromat. Compd. 2014, 34, 397–424. [Google Scholar] [CrossRef]
- Harris, K.A.; Yunker, M.B.; Dangerfield, N.; Ross, P.S. Sediment-associated aliphatic and aromatic hydrocarbons in coastal British Columbia, Canada: Concentrations, composition, and associated risks to protected sea otters. Environ. Pollut. 2011, 159, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xie, Z.; Yang, H.; Moeller, A.; Halsall, C.; Cai, M.; Sturm, R.; Ebinghaus, R. Deposition of polycyclic aromatic hydrocarbons in the North Pacific and the arctic. J. Geophys. Res.-Atmos. 2013, 118, 5822–5829. [Google Scholar] [CrossRef]
- Yim, U.H.; Hong, S.H.; Shim, W.J.; Oh, J.R.; Chang, M. Spatio-temporal distribution and characteristics of PAHs in sediments from Masan Bay, Korea. Mar. Pollut. Bull. 2005, 50, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernandez, A.C.; Sprovieri, M.; Frignani, M.; Sanchez-Cabeza, J.A.; Feo, M.L.; Bellucci, L.G.; Perez-Bernal, L.H.; Preda, M.; Machain-Castillo, M.L. Reconstruction of hydrocarbons accumulation in sediments affected by the oil refinery industry: The case of Tehuantepec Gulf (Mexico). Environ. Earth Sci. 2012, 67, 727–742. [Google Scholar] [CrossRef]
- Ke, H.W.; Chen, M.; Liu, M.Y.; Chen, M.; Duan, M.S.; Huang, P.; Hong, J.J.; Lin, Y.; Cheng, S.Y.; Wang, X.R.; et al. Fate of polycyclic aromatic hydrocarbons from the north pacific to the arctic: Field measurements and fugacity model simulation. Chemosphere 2017, 184, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Macdonald, R.W.; Jantunen, L.M.M.; Harner, T.; Bidleman, T.F.; Strachan, W.M.J. The transport of beta-hexachlorocyclohexane to the western Arctic Ocean: A contrast to alpha-HCH. Sci. Total Environ. 2002, 291, 229–246. [Google Scholar] [CrossRef]
- Qiu, C.; Cai, M. Ultra trace analysis of 17 organochlorine pesticides in water samples from the Arctic based on the combination of solid-phase extraction and headspace solid-phase microextraction–gas chromatography-electron-capture detector. J. Chromatogr. A 2010, 1217, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Na, G.; Ma, X.; Fang, X.; Ge, L.; Gao, H.; Yao, Z. Occurrence and gas/particle partitioning of PAHs in the atmosphere from the North Pacific to the Arctic Ocean. Atmos. Environ. 2013, 77, 640–646. [Google Scholar] [CrossRef]
- Wu, X.G.; Lam, J.C.W.; Xia, C.H.; Kang, H.; Sun, L.G.; Xie, Z.Q.; Lam, P.K.S. Atmospheric HCH Concentrations over the Marine Boundary Layer from Shanghai, China to the Arctic Ocean: Role of Human Activity and Climate Change. Environ. Sci. Technol. 2010, 44, 8422–8428. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.C.; Xie, Z.Y.; Cai, M.H.; Moller, A.; Sturm, R.; Tang, J.H.; Zhang, G.; He, J.F.; Ebinghaus, R. Distribution and Air-Sea Exchange of Current-Use Pesticides (CUPs) from East Asia to the High Arctic Ocean. Environ. Sci. Technol. 2012, 46, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.; Blanchard, P.; Halsall, C.J.; Bidleman, T.F.; Stern, G.A.; Fellin, P.; Muir, D.C.G.; Barrie, L.A.; Jantunen, L.M.; Helm, P.A. Temporal and spatial variabilities of atmospheric polychlorinated biphenyls (PCBs), organochlorine (OC) pesticides and polycyclic aromatic hydrocarbons (PAHs) in the Canadian Arctic: Results from a decade of monitoring. Sci. Total Environ. 2005, 342, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Savinov, V.M.; Savinova, T.N.; Matishov, G.G.; Dahle, S.; Naes, K. Polycyclic aromatic hydrocarbons (PAHs) and organochlorines (OCs) in bottom sediments of the Guba Pechenga, Barents Sea, Russia. Sci. Total Environ. 2003, 306, 39–56. [Google Scholar] [CrossRef]
- Sericano, J.L.; Brooks, J.M.; Champ, M.A.; Ii, M.C.K.; Makeyev, V.V. Trace Contaminant Concentrations in the Kara Sea and its Adjacent Rivers, Russia. Mar. Pollut. Bull. 1998, 42, 1017–1030. [Google Scholar] [CrossRef]
- Evenset, A.; Christensen, G.N.; Carroll, J.; Zaborska, A.; Berger, U.; Herzke, D.; Gregor, D. Historical trends in persistent organic pollutants and metals recorded in sediment from Lake Ellasjøen, Bjørnøya, Norwegian Arctic. Environ. Pollut. 2007, 146, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.P.; Zheng, G.J.; Tu, B.M.; Richardson, B.; Chen, L.; Zhang, Y.H.; Yeung, L.W.; Lam, J.C.W.; Yang, X.L.; Lam, P.K.S. Persistent toxic substances in remote lake and coastal sediments from Svalbard, Norwegian Arctic: Levels, sources and fluxes. Environ. Pollut. 2009, 157, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Sapota, G.; Wojtasik, B.; Burska, D.; Nowin´Ski, K. Persistent organic pollutants (POPs) and polycyclic aromatic hydrocarbons (PAHs) in surface sediments from selected fjords, tidal plains and lakes of the North Spitsbergen. Pol. Polar Res. 2009, 30, 59–76. [Google Scholar]
- Yunker, M.B.; Macdonald, R.W.; Cretney, W.J.; Fowler, B.R.; McLaughlin, F.A. Alkane, terpene, and polycyclic aromatic hydrocarbon geochemistry of the mackenzie river and mackenzie shelf-riverine contributions to beaufort sea coastal sediment. Geochim. Cosmochim. Acta 1993, 57, 3041–3061. [Google Scholar] [CrossRef]
- Kuzyk, Z.A.; Stow, J.P.; Burgess, N.M.; Solomon, S.M.; Reimer, K.J. PCBs in sediments and the coastal food web near a local contaminant source in Saglek Bay, Labrador. Sci. Total Environ. 2005, 351, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Tanabe, S.; Aramoto, M.; Sakai, N.; Tatsukawa, R. Persistent organochlorine residues in sediments from the Chukchi Sea, Bering Sea And Gulf of Alaska. Mar. Pollut. Bull. 1994, 28, 746–753. [Google Scholar] [CrossRef]
- Cai, M.; Lin, Y.; Chen, M.; Yang, W.; Du, H.; Xu, Y.; Cheng, S.; Xu, F.; Hong, J.; Chen, M. Improved source apportionment of PAHs and Pb by integrating Pb stable isotopes and positive matrix factorization application (PAHs): A historical record case study from the northern South China Sea. Sci. Total Environ. 2017, 609, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.B.; Macdonald, R.W. Composition and origins of polycyclic aromatic-hydrocarbons in the Mackenzie River and on the Beaufort Sea shelf. Arctic 1995, 48, 118–129. [Google Scholar] [CrossRef]
- Valette-Silver, N.; Jawed Hameedi, M.; Efurd, D.W.; Robertson, A. Status of the Contamination in Sediments and Biota from the Western Beaufort Sea (Alaska). Mar. Pollut. Bull. 1999, 38, 702–722. [Google Scholar] [CrossRef]
- Venkatesan, M.I.; Naidu, A.S.; Blanchard, A.L.; Misra, D.; Kelley, J.J. Historical changes in trace metals and hydrocarbons in nearshore sediments, Alaskan Beaufort Sea, prior and subsequent to petroleum-related industrial development: Part II. Hydrocarbons. Mar. Pollut. Bull. 2013, 77, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.R.; Taylor, K.A.; Pie, H.V.; Mitchelmore, C.L. Polycyclic aromatic and aliphatic hydrocarbons in Chukchi Sea biota and sediments and their toxicological response in the Arctic cod, Boreogadus saida. Deep-Sea Res. Part II-Top. Stud. Oceanogr. 2014, 102, 32–55. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Snowdon, L.R.; Fowler, B.R. Alkane and PAH biomarkers as tracers of terrigenous organic carbon in Arctic Ocean sediments. Org. Geochem. 2011, 42, 1109–1146. [Google Scholar] [CrossRef]
- Guinan, J.; Charlesworth, M.; Service, M.; Oliver, T. Sources and Geochemical Constraints of Polycyclic Aromatic Hydrocarbons (PAHs) in Sediments and Mussels of two Northern Irish Sea-loughs. Mar. Pollut. Bull. 2001, 42, 1073–1081. [Google Scholar] [CrossRef]
- Cornelissen, G.; Pettersen, A.; Nesse, E.; Eek, E.; Helland, A.; Breedveld, G. The contribution of urban runoff to organic contaminant levels in harbour sediments near two Norwegian cities. Mar. Pollut. Bull. 2008, 56, 565–573. [Google Scholar] [CrossRef] [PubMed]
- NæS, K.; Oug, E. The distribution and environmental relationships of polycyclic aromatic hydrocarbons (PAHs) in sediments from Norwegian smelter-affected fjords. Chemosphere 1998, 36, 561–576. [Google Scholar] [CrossRef]
- Savinov, V.M.; Savinova, T.N.; Carroll, J.L.; Matishov, G.G.; Dahle, S.; Næs, K. Polycyclic aromatic hydrocarbons (PAHs) in sediments of the White Sea, Russia. Mar. Pollut. Bull. 2000, 40, 807–818. [Google Scholar] [CrossRef]
- Fernandes, M.; Sicre, M.A. Polycyclic aromatic hydrocarbons in the Arctic: Ob and Yenisei estuaries and Kara Sea shelf. Estuar. Coast. Shelf Sci. 1999, 48, 725–737. [Google Scholar] [CrossRef]
- Boitsov, S.; Jensen, H.K.B.; Klungsøyr, J. Natural background and anthropogenic inputs of polycyclic aromatic hydrocarbons (PAH) in sediments of South-Western Barents Sea. Mar. Environ. Res. 2009, 68, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Blodau, C.; Turunen, J.; Radke, M. The spatial distribution of pah depositions to peatlands of Eastern Canada. Atmos. Environ. 2005, 39, 3725–3733. [Google Scholar] [CrossRef]
- Charlesworth, M. Pah contamination of western Irish Sea sediments. Mar. Pollut. Bull. 2002, 44, 1421–1426. [Google Scholar] [CrossRef]
- Wayland, M.; Headley, J.V.; Peru, K.M.; Crosley, R.; Brownlee, B.G. Levels of polycyclic aromatic hydrocarbons and dibenzothiophenes in wetland sediments and aquatic insects in the oil sands area of Northeastern Alberta, Canada. Environ. Monit. Assess. 2007, 136, 167–182. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.W.; Barrie, L.A.; Bidleman, T.F.; Diamond, M.L.; Gregor, D.J.; Semkin, R.G.; Strachan, W.M.J.; Li, Y.F.; Wania, F.; Alaee, M.; et al. Contaminants in the Canadian arctic: 5 Years of progress in understanding sources, occurrence and pathways. Sci. Total Environ. 2000, 254, 93–234. [Google Scholar] [CrossRef]
- Macdonald, R.W.; Solomon, S.M.; Cranston, R.E.; Welch, H.E.; Yunker, M.B.; Gobeil, C. A sediment and organic carbon budget for the Canadian Beaufort shelf. Mar. Geol. 1998, 144, 255–273. [Google Scholar] [CrossRef]
- McRae, C.; Snape, C.E.; Sun, C.G.; Fabbri, D.; Tartari, D.; Trombini, C.; Fallick, A.E. Use of compound-specific stable isotope analysis to source anthropogenic natural gas-derived polycyclic aromatic hydrocarbons in a lagoon sediment. Environ. Sci. Technol. 2000, 34, 4684–4686. [Google Scholar] [CrossRef]
- Zou, Y.H.; Wang, L.X.; Christensen, E.R. Problems in the fingerprints based polycyclic aromatic hydrocarbons source apportionment analysis and a practical solution. Environ. Pollut. 2015, 205, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xue, Y.; Leeuw, G.D.; Guang, J.; Wang, Y.; Li, Y.; Xu, H.; Yang, L.; Hou, T.; He, X. Corrigendum to “Integration of remote sensing data and surface observations to estimate the impact of the Russian wildfires over Europe and Asia during August 2010”. Biogeosciences 2011, 9, 3771–3791. [Google Scholar] [CrossRef] [Green Version]
- Hinga, K.R. Degradation rates of low molecular weight PAH correlate with sediment TOC in marine subtidal sediments. Mar. Pollut. Bull. 2003, 46, 466–474. [Google Scholar] [CrossRef]
- Rothermich, M.M.; Hayes, L.A.; Lovley, D.R. Anaerobic, sulfate-Dependent Degradation of Polycyclic Aromatic Hydrocarbons in Petroleum-Contaminated Harbor Sediment. Environ. Sci. Technol. 2002, 36, 4811–4817. [Google Scholar] [CrossRef] [PubMed]
- Masclet, P.; Mouvier, G.; Nikolaou, K. Relative decay index and sources of polycyclic aromatic hydrocarbons. Atmos. Environ. (1967) 1986, 20, 439–446. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuary sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and evaluation of sediment quality guidelines for florida coastal waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef] [PubMed]




| Region | Number | ∑PAHs (ng/g d.w.) | Reference |
|---|---|---|---|
| Bering Sea | 16 | 49.84–65.38 | This Study |
| Chukchi Sea | 16 | 52.40–91.25 | |
| Canadian Basin | 16 | 27.66–167.48 | |
| Western Beaufort Sea (Alaska) | 6 | 159–1092 | [51] |
| NS&T Alaska | 6 | 2.17–733 | |
| Barent Sea | - | 1500 | [41] |
| Cork Harbour in south east in Ireland | 21 | 924–2877 | [11] |
| Northern Ireland lake | - | 83~2300 | [55] |
| Harbor (Oslo and Drammen), Norway | 16 | 3000–4800 | [56] |
| Ardal Fjords, Norway | 13 | 45,565–784,296 | [57] |
| Ny-Ålesund, Svalbard, Norway S1 | 15 | 34 | [44] |
| Ny-Ålesund, Svalbard, Norway S2 | 15 | 27 | |
| White Sea, Russia | 27 | 27–95 | [58] |
| White Sea, Russia | 27 | 13–208 | [58] |
| Ob | 12 | 24–115 | [59] |
| Yenisei | 12 | 40–131 | |
| Kara Sea | 12 | 16–94 | |
| Northern South China Sea | 15 | 11.3–95.5 | [49] |
| Fjord areas, Barents | 28 | 209–326 | [60] |
| Tromsoflaket, Barents | 28 | 58.8–224 | |
| Ingoydjupet, Barents | 28 | 157–217 | |
| Eastern Canada | 13 | 48–2790 | [61] |
| Western areas in Ireland | 15 | 100–1422 | [62] |
| Northeastern Alberta, Canada | 35 | ND-3900 | [63] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Lin, Y.; Cai, M.; Zhang, J.; Zhang, Y.; Kuang, W.; Liu, L.; Huang, P.; Ke, H. Occurrence and Risk Assessment of PAHs in Surface Sediments from Western Arctic and Subarctic Oceans. Int. J. Environ. Res. Public Health 2018, 15, 734. https://doi.org/10.3390/ijerph15040734
Chen F, Lin Y, Cai M, Zhang J, Zhang Y, Kuang W, Liu L, Huang P, Ke H. Occurrence and Risk Assessment of PAHs in Surface Sediments from Western Arctic and Subarctic Oceans. International Journal of Environmental Research and Public Health. 2018; 15(4):734. https://doi.org/10.3390/ijerph15040734
Chicago/Turabian StyleChen, Fajin, Yan Lin, Minggang Cai, Jingjing Zhang, Yuanbiao Zhang, Weiming Kuang, Lin Liu, Peng Huang, and Hongwei Ke. 2018. "Occurrence and Risk Assessment of PAHs in Surface Sediments from Western Arctic and Subarctic Oceans" International Journal of Environmental Research and Public Health 15, no. 4: 734. https://doi.org/10.3390/ijerph15040734
APA StyleChen, F., Lin, Y., Cai, M., Zhang, J., Zhang, Y., Kuang, W., Liu, L., Huang, P., & Ke, H. (2018). Occurrence and Risk Assessment of PAHs in Surface Sediments from Western Arctic and Subarctic Oceans. International Journal of Environmental Research and Public Health, 15(4), 734. https://doi.org/10.3390/ijerph15040734