Application of Chemical Crystallization Circulating Pellet Fluidized Beds for Softening and Saving Circulating Water in Thermal Power Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
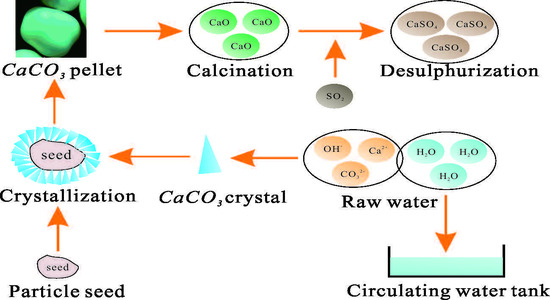
2.2. Full-Scale Experimental System and Process Description
2.3. Analytical Methods
3. Results and Discussion
3.1. Softening Performance of the CPFBs for Replenishment of Circulating Water
3.1.1. Efficiency of CPFBs for Reducing Hardness
3.1.2. Efficiency of CPFBs for Reducing Alkalinity
3.1.3. Composition Analysis of Particles Discharged from the CPFBs
3.2. Application of a CPFB System for Water Saving and Emission Reduction in Power Plants
3.2.1. Dynamic Simulation Test on the Circulating Water
3.2.2. Effect of Water Discharged from Circulating Pellet Fluidized Bed on Dosage of Scale Inhibitors
3.2.3. Impact of High Concentration Ratio on Promoting Zero Emissions in the Entire Plant
3.3. Evaluation of Economic and Environmental Benefits of CPFBs System
4. Conclusions
- (1)
- The application of circulating pellet fluidized beds to soften the circulating water in the Dingzhou Power Plant results in a hardness removal rate of 40%–50% and a Ca2+ ion removal of 90%, both of which ensure stable quality of the softened water. In addition, CaO contents in the discharged particles are greater than 50%, which allows these particles to be used directly in the desulfurization system in the power plant. Therefore, no wastewater or waste solids are generated from the entire system.
- (2)
- By pretreating the water via the use of circulating pellet fluidized beds and by discharging it into the circulating water system in the Dingzhou Power Plant, the concentration ratio of the circulating water is increased from 4.5 to 9.2, the amount of replenishing water and wastewater discharges are both reduced by 150 m3/h, and the dosage of the scale inhibitor is reduced by more than 30%.
- (3)
- The application of the circulating crystallization pellet fluidized bed system to soften the circulating water in the Dingzhou Power Plant reduces the cost of treating the circulating water to only 0.072 US$/m3. The power plant can thus save as much as 200,000 dollars per year. Therefore, the proposed softening technology can provide significant economic, environmental, and social benefits.
Author Contributions
Funding
Conflicts of Interest
References
- Shao, W.; Feng, J.; Liu, J.; Yang, G.; Yang, Z.; Wang, J. Research on the Status of Water Conservation in the Thermal Power Industry in China. Energy Procedia 2017, 105, 3068–3074. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S.; Versteeg, P.L. Water Use at Pulverized Coal Power Plants with Postcombustion Carbon Capture and Storage. Environ. Sci. Technol. 2011, 45, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Tang, Y.; Zhao, X.; Yang, H.; Gerbens-Leenes, P.W.; van Vliet, M.T.H.; Yan, J. China’s coal-fired power plants impose pressure on water resources. J. Clean. Prod. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Regucki, P.; Lewkowicz, M.; Krzyżyńska, R.; Jouhara, H. Numerical study of water flow rates in power plant cooling systems. Therm. Sci. Eng. Prog. 2018, 7, 27–32. [Google Scholar] [CrossRef]
- Yi, L.; Jiao, W.; Chen, X.; Chen, W. An overview of reclaimed water reuse in China. J. Environ. Sci. 2011, 23, 1585–1593. [Google Scholar] [CrossRef]
- Rahmani, K. Reducing water consumption by increasing the cycles of concentration and Considerations of corrosion and scaling in a cooling system. Appl. Therm. Eng. 2017, 114, 849–856. [Google Scholar] [CrossRef]
- Ochoa, N.; Baril, G.; Moran, F.; Pébère, N. Study of the properties of a multi-component inhibitor used for water treatment in cooling circuits. J. Appl. Electrochem. 2002, 32, 497–504. [Google Scholar] [CrossRef]
- Rahmani, K.; Jadidian, R.; Haghtalab, S. Evaluation of inhibitors and biocides on the corrosion, scaling and biofouling control of carbon steel and copper–nickel alloys in a power plant cooling water system. Desalination 2016, 393, 174–185. [Google Scholar] [CrossRef]
- Vahedi, A.; Gorczyca, B. Application of fractal dimensions to study the structure of flocs formed in lime softening process. Water Res. 2011, 45, 545–556. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.J.; Lytle, D.A.; Harmon, S.; Vu, K.; Chait, H.; Dionysiou, D.D. Removal of strontium from drinking water by conventional treatment and lime softening in bench-scale studies. Water Res. 2016, 103, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Huang, T.; Zhi, A.; Tang, Z. Full-Scale Experimental Study of Groundwater Softening in a Circulating Pellet Fluidized Reactor. Intern. J. Environ. Res. Public Health 2018, 15, 1592. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.Z.; Huang, T.L.; Wen, G.; Yang, S.Y. Modelling particle growth of calcium carbonate in a pilot-scale pellet fluidized bed reactor. Water Sci. Technol. Water Supply 2016, 17, 643–651. [Google Scholar] [CrossRef]
- Tang, Z.C.; Huang, T.L.; Hu, R.Z.; Zhang, R.F. Experimental study on softening high permanent hardness water in thermal power plants by induced crystallization. Water Treat. Technol. 2019, 45, 33–37, 46. [Google Scholar]
- HG/T 2160-2008. Dynamic Simulation Test Method for Cooling Water; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- GB/T50050-2017. Code for Design of Industrial Circulating Cooling Water Treatment; Planning Publishing House: Beijing, China, 2017. [Google Scholar]
- GB/T18175-2014. Determination of Corrosion Inhibition Performance of Water Treatment Agents-Rotation Specimen Method; Standards Publishing House: Beijing, China, 2014. [Google Scholar]
- Jiang, K.; Zhou, K.G.; Yang, Y.C.; Du, H. Growth kinetics of calcium fluoride at high supersaturation in a fluidized bed reactor. Environ. Technol. 2014, 35, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.Y. Crystal growth kinetics of two-step growth process in liquid Fluidized-bed crystallizers. J. Cryst. Growth 1999, 206, 109–118. [Google Scholar] [CrossRef]
- Schetters, M.J.A.; Van Der Hoek, J.P.; Kramer, O.J.I.; Kors, L.J.; Palmen, L.J.; Hofs, B.; Koppers, H. Circular economy in drinking water treatment: Reuse of ground pellets as seeding material in the pellet softening process. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2015, 71, 479. [Google Scholar] [CrossRef] [PubMed]
- Scala, F.; Chirone, R.; Meloni, P.; Carcangiu, G.; Manca, M.; Mulas, G.; Mulas, A. Fluidized bed desulfurization using lime obtained after slow calcination of limestone particles. Fuel 2013, 114, 99–105. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, R.; An, D.; Cheng, Y.; Tan, H. Water softening by induced crystallization in fluidized bed. J. Environ. Sci. Engl. Ed. 2016, 50, 109. [Google Scholar] [CrossRef] [PubMed]
- DB13T 2032-2014. Limestone Powder for Flue Gas Desulfurization (Wet Process); Hebei Quality and Supervision Bureau: Hebei, China, 2014.
- Liu, X.; Geng, M.; Zhou, S. Study on the scale and corrosion inhibitor of urban reclaimed water reused in circulating cooling water of power plant. In Proceedings of the 3rd International Conference on Energy Engineering and Environmental Protection, EEEP 2018, Sanya, China, 19–21 November 2018. [Google Scholar]
- Xu, J.; Lin, K.; Huang, Y.; Guo, Q.; Li, H.; Yuan, D. Development of an online analyzer for determination of total phosphorus in industrial circulating cooling water with UV photooxidation digestion and spectrophotometric detection. Talanta 2019, 201, 74–81. [Google Scholar] [CrossRef] [PubMed]







| pH | Turbidity (NTU) | Total Alkalinity (mM) | HCO3− (mM) | Total Hardness (mM) | Ca2+ (mM) | Mg2+ (mM) |
|---|---|---|---|---|---|---|
| 7.5–7.7 | <1 | 3.1–3.5 | 3.1–3.5 | 2.3–2.75 | 1.6–1.75 | 0.7–1.0 |
| Items | MgO | Al2O3 | SiO2 | K2O | MnO2 | Fe2O3 | CaO |
|---|---|---|---|---|---|---|---|
| (Determined Based on the Oxide Product of Different Elements) | |||||||
| Analysis results | 0.74% | 0.07% | 2.99% | 0.09% | 0.06% | 1.33% | 51.10% |
| Water Sample | Klimit | 85% Klimit | Cl−limit (mg/L) | Ca2+limit (mg/L) | ΔB | Note |
|---|---|---|---|---|---|---|
| Water discharged from the CPFBR system | 10.82 | 9.20 | 238 | 92.58 | −0.20 | Chemicals were added to the water sample up to a concentration of 8.5 mg/L; acid was added to the water sample to control the p-alkalinity (≤1.0 mM) |
| Indicator | Concentration Ratio | Calcium Ion (mg/L) | Hardness (mM) | P-Alkalinity (mM) | Total Alkalinity (mM) | pH |
|---|---|---|---|---|---|---|
| Circulating water | ≤9.20 | ≤77.28 | ≤20.08 | ≤1.0 | ≤6.6 | ≤8.75 |
| Operating Conditions | Klimit | 85% Klimit | Cl−limit (mg/L) | Ca2+limit (mg/L) | ΔB | Control Conditions |
|---|---|---|---|---|---|---|
| 1 | 6.64 | 5.64 | 146 | 52.99 | 0.19 | Scale inhibitor concentration = 8.5 mg/L |
| 2 | 10.91 | 9.27 | 240 | 91.18 | −0.18 | Scale inhibitor concentration = 6.0 mg/L, p-alkalinity ≤ 1.0 mM, controlled by adding acid |
| Number | Indicator | Quantity | Unit Cost | Economic Benefit (dollars per year) | Note |
|---|---|---|---|---|---|
| 1 | Reduction in replenishing water (m3/h) | 150 | $0.398 per m3 | 480977 | Operating for 335 days a year |
| 2 | Reduction in waste discharge (m3/h) | 150 | $0.072 per m3 | 87134 | Operating for 335 days a year |
| 3 | Reduction in scale inhibitor dosage (mg/L) | 2.5 | $1.156 per ton | 22499 | Average water usage rate = 1000 m3/h |
| 4 | Amount of water replenished to the circulating water (m3/h) | 1050 | $0.072 per m3 | 390150 | Current real water volume 6,722,400 m3/y |
| 5 | Cost savings per year | 200459 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Huang, T.; Wang, T.; Wang, H.; Long, X. Application of Chemical Crystallization Circulating Pellet Fluidized Beds for Softening and Saving Circulating Water in Thermal Power Plants. Int. J. Environ. Res. Public Health 2019, 16, 4576. https://doi.org/10.3390/ijerph16224576
Hu R, Huang T, Wang T, Wang H, Long X. Application of Chemical Crystallization Circulating Pellet Fluidized Beds for Softening and Saving Circulating Water in Thermal Power Plants. International Journal of Environmental Research and Public Health. 2019; 16(22):4576. https://doi.org/10.3390/ijerph16224576
Chicago/Turabian StyleHu, Ruizhu, Tinglin Huang, Tianwei Wang, Huixin Wang, and Xiao Long. 2019. "Application of Chemical Crystallization Circulating Pellet Fluidized Beds for Softening and Saving Circulating Water in Thermal Power Plants" International Journal of Environmental Research and Public Health 16, no. 22: 4576. https://doi.org/10.3390/ijerph16224576
APA StyleHu, R., Huang, T., Wang, T., Wang, H., & Long, X. (2019). Application of Chemical Crystallization Circulating Pellet Fluidized Beds for Softening and Saving Circulating Water in Thermal Power Plants. International Journal of Environmental Research and Public Health, 16(22), 4576. https://doi.org/10.3390/ijerph16224576