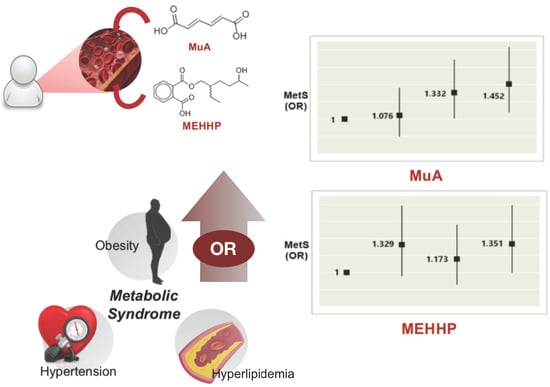
Association between Heavy Metals, Bisphenol A, Volatile Organic Compounds and Phthalates and Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Demographic Variables
2.2. Environmental Hazardous Material Concentrations
2.3. Definition of MetS
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants According to MetS Status
3.2. Differences in Log-Transformed Blood and Urine Hazardous Material Concentrations by MetS Status
3.3. Multiple Logistic Regression Analysis between MetS Status and Environmental Hazardous Material Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, A.; Wu, L.; Liu, X.; Su, Z.; Luo, Y.; Chen, S.; Li, H.; Liu, X.; Tao, L.; Guo, J.; et al. The prevalence of carotid plaque with different stability and its association with metabolic syndrome in China: The Asymptomatic Polyvascular Abnormalities Community study. Medicine 2016, 95, e4619. [Google Scholar] [CrossRef] [PubMed]
- Molokwu, J.C.; Penaranda, E.; Lopez, D.S.; Dwivedi, A.; Dodoo, C.; Shokar, N. Association of Metabolic Syndrome and Human Papillomavirus Infection in Men and Women Residing in the United States. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2017, 26, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Liu, F.; Wang, C.; Liang, K.; Yan, F.; Hou, X.; Liu, J.; Chen, L. Both insulin resistance and metabolic syndrome accelerate the progression of chronic kidney disease among Chinese adults: Results from a 3-year follow-up study. Int. Urol. Nephrol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, H.J.; Oh, H.J.; Go, T.; Kang, D.R.; Kim, J.Y.; Huh, J.H. Metabolic syndrome status over 2 years predicts incident chronic kidney disease in mid-life adults: A 10-year prospective cohort study. Sci. Rep. 2018, 8, 12237. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Braithwaite, D.; Akinyemiju, T. Metabolic Syndrome and the Risk of Breast Cancer and Subtypes by Race, Menopause and BMI. Cancers 2018, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Farre, P.L.; Scalise, G.D.; Duca, R.B.; Dalton, G.N.; Massillo, C.; Porretti, J.; Grana, K.; Gardner, K.; De Luca, P.; De Siervi, A. CTBP1 and metabolic syndrome induce an mRNA and miRNA expression profile critical for breast cancer progression and metastasis. Oncotarget 2018, 9, 13848–13858. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.E.; Kim, S.M.; Han, K.; Kim, N.H.; Chung, H.S.; Kim, J.W.; Han, B.; Cho, S.J.; Yu, J.H.; Park, Y.G.; et al. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. 2018, 15, e1002640. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kang, J.G.; Han, J.M.; Kim, J.H.; Lee, S.J.; Seo, D.C.; Lee, S.H.; Kim, B.S.; Kang, J.H. Association of self-reported and cotinine-verified smoking status with incidence of metabolic syndrome in 47,379 Korean Adults. J. Diabetes 2018. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Ng, A.H.; Wan, H.T.; Wong, A.Y.; Leung, C.C.; Li, R.; Wong, C.K. Dietary Exposure to the Environmental Chemical, PFOS on the Diversity of Gut Microbiota, Associated With the Development of Metabolic Syndrome. Front. Microbiol. 2018, 9, 2552. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.S. Association of lead, mercury and cadmium with diabetes in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabet. Med. J. Br. Diabet. Assoc. 2013, 30, e143–e148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mukherjee, B.; Park, S.K. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 2018, 121, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Hwang, Y.C.; Woo, J.T.; Sinn, D.H.; Chin, S.O.; Chon, S.; Kim, Y.S. Blood lead is significantly associated with metabolic syndrome in Korean adults: An analysis based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. Cardiovasc. Diabetol. 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, Y. Blood cadmium, mercury, and lead and metabolic syndrome in South Korea: 2005–2010 Korean National Health and Nutrition Examination Survey. Am. J. Ind. Med. 2013, 56, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. Blood mercury concentration in relation to metabolic and weight phenotypes using the KNHANES 2011–2013 data. Int. Arch. Occup. Environ. Health 2018, 91, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.Y.; Choi, S.H.; Ahn, S.J.; Kim, D.K.; Kim, D.W.; Lim, J.A.; Choi, B.S.; Shin, H.J.; Yun, S.W.; Yoon, H.J.; et al. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int. Arch. Occup. Environ. Health 2014, 87, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Piecha, R.; Svacina, S.; Maly, M.; Vrbik, K.; Lacinova, Z.; Haluzik, M.; Pavlouskova, J.; Vavrous, A.; Matejkova, D.; Mullerova, D.; et al. Urine Levels of Phthalate Metabolites and Bisphenol A in Relation to Main Metabolic Syndrome Components: Dyslipidemia, Hypertension and Type 2 Diabetes. A Pilot Study. Cent. Eur. J. Public Health 2016, 24, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: A population-based cross-sectional study. Int. J. Hyg. Environ. Health 2013, 216, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.K.; O’Neill, M.S.; Sowers, M.R.; Park, S.K. Urinary bisphenol A and type-2 diabetes in U.S. adults: Data from NHANES 2003–2008. PLoS ONE 2011, 6, e26868. [Google Scholar] [CrossRef] [PubMed]
- Ahmadkhaniha, R.; Mansouri, M.; Yunesian, M.; Omidfar, K.; Jeddi, M.Z.; Larijani, B.; Mesdaghinia, A.; Rastkari, N. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J. Environ. Health Sci. Eng. 2014, 12, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andra, S.S.; Kalyvas, H.; Andrianou, X.D.; Charisiadis, P.; Christophi, C.A.; Makris, K.C. Preliminary evidence of the association between monochlorinated bisphenol A exposure and type II diabetes mellitus: A pilot study. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.E.; Nelson, J.W.; Qureshi, M.M.; Weinberg, J.; Moore, L.L.; Singer, M.; Webster, T.F. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A cross-sectional study of NHANES data, 1999–2002. Environ. Health Glob. Access Sci. Source 2008, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- James-Todd, T.; Stahlhut, R.; Meeker, J.D.; Powell, S.G.; Hauser, R.; Huang, T.; Rich-Edwards, J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. 2012, 120, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, R.W.; van Wijngaarden, E.; Dye, T.D.; Cook, S.; Swan, S.H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Attina, T.M. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension 2015, 66, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gaston, S.A.; Tulve, N.S. Urinary phthalate metabolites and metabolic syndrome in U.S. adolescents: Cross-sectional results from the National Health and Nutrition Examination Survey (2003–2014) data. Int. J. Hyg. Environ. Health 2018. [Google Scholar] [CrossRef] [PubMed]
- James-Todd, T.M.; Huang, T.; Seely, E.W.; Saxena, A.R. The association between phthalates and metabolic syndrome: The National Health and Nutrition Examination Survey 2001–2010. Environ. Health Glob. Access Sci. Source 2016, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. A portion of plant airborne communication is endorsed by uptake and metabolism of volatile organic compounds. Curr. Opin. Plant Biol. 2016, 32, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, H.; Ong, C.N.; Patra, A.; Lu, Y.; Lim, C.T.; Venkatesan, T. Detection of Lung Cancer: Concomitant Volatile Organic Compounds and Metabolomic Profiling of Six Cancer Cell Lines of Different Histological Origins. ACS Omega 2018, 3, 5131–5140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.C.; Wu, S.Y.; Ke, Y.B. [Association of exposure to environmental chemicals with risk of childhood acute lymphocytic leukemia]. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2016, 50, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Abplanalp, W.; DeJarnett, N.; Riggs, D.W.; Conklin, D.J.; McCracken, J.P.; Srivastava, S.; Xie, Z.; Rai, S.; Bhatnagar, A.; O’Toole, T.E. Benzene exposure is associated with cardiovascular disease risk. PLoS ONE 2017, 12, e0183602. [Google Scholar] [CrossRef] [PubMed]
- De Nys, S.; Putzeys, E.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L.; et al. A novel high sensitivity UPLC-MS/MS method for the evaluation of bisphenol A leaching from dental materials. Sci. Rep. 2018, 8, 6981. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Malek, N.A.; Hodge, C.C.; Reidy, J.A.; Kato, K.; Barr, D.B.; Needham, L.L.; Brock, J.W. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 789, 393–404. [Google Scholar] [CrossRef]
- Park, H.S.; Park, C.Y.; Oh, S.W.; Yoo, H.J. Prevalence of obesity and metabolic syndrome in Korean adults. Obes. Rev. Off. J. Int. Assoc. Stud. Obes. 2008, 9, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Zong, G.; Seely, E.W.; Weisskopf, M.; James-Todd, T. Urinary cadmium concentrations and metabolic syndrome in U.S. adults: The National Health and Nutrition Examination Survey 2001–2014. Environ. Int. 2018, 121, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.; Garte, S.; Barchowsky, A.; Zmuda, J.; Taioli, E. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 polymorphisms and biological effects of benzene exposure—A literature review. Toxicol. Lett. 2008, 182, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Kim, S.; Lan, Q.; Li, G.; Vermeulen, R.; Waidyanatha, S.; Zhang, L.; Yin, S.; Smith, M.T.; Rothman, N. Human benzene metabolism following occupational and environmental exposures. Chemico-Biol. Interact. 2010, 184, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansi, A.; Bruni, R.; Capone, P.; Paci, E.; Pigini, D.; Simeoni, C.; Gnerre, R.; Papacchini, M.; Tranfo, G. Low occupational exposure to benzene in a petrochemical plant: Modulating effect of genetic polymorphisms and smoking habit on the urinary t,t-MA/SPMA ratio. Toxicol. Lett. 2012, 213, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Langard, S.; Lin, Y.S. A critique of benzene exposure in the general population. Sci. Total Environ. 2007, 374, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Janitz, A.E.; Campbell, J.E.; Magzamen, S.; Pate, A.; Stoner, J.A.; Peck, J.D. Corrigendum to ‘Benzene and childhood acute leukemia in Oklahoma’. Environ. Res. 2018, 165, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Vlaanderen, J.; Lan, Q.; Kromhout, H.; Rothman, N.; Vermeulen, R. Occupational benzene exposure and the risk of chronic myeloid leukemia: A meta-analysis of cohort studies incorporating study quality dimensions. Am. J. Ind. Med. 2012, 55, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Khalade, A.; Jaakkola, M.S.; Pukkala, E.; Jaakkola, J.J. Exposure to benzene at work and the risk of leukemia: A systematic review and meta-analysis. Environ. Health Glob. Access Sci. Source 2010, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Autrup, H.; Moller, P.; Hertel, O.; Jensen, S.S.; Vinzents, P.; Knudsen, L.E.; Loft, S. Linking exposure to environmental pollutants with biological effects. Mutat. Res. 2003, 544, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Madureira, D.; Capani, F.; Aon-Bertolino, L.; Saraceno, E.; Alvarez-Giraldez, L.D. The role of catechols and free radicals in benzene toxicity: An oxidative DNA damage pathway. Environ. Mol. Mutagen. 2009, 50, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, J.H.; Lee, B.E.; Hong, Y.C. Urinary benzene metabolite and insulin resistance in elderly adults. Sci. Total Environ. 2014, 482–483, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barcelo, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Holst, C.; Becker, U.; Jorgensen, M.E.; Gronbaek, M.; Tolstrup, J.S. Alcohol drinking patterns and risk of diabetes: A cohort study of 70,551 men and women from the general Danish population. Diabetologia 2017, 60, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [PubMed]
- Brautbar, A.; Leary, E.; Rasmussen, K.; Wilson, D.P.; Steiner, R.D.; Virani, S. Genetics of familial hypercholesterolemia. Curr. Atheroscler. Rep. 2015, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, S.J.; Molloy, P.L.; Varinli, H.; Morrison, J.L.; Muhlhausler, B.S. Epigenetics and human obesity. Int. J. Obes. 2015, 39, 85–97. [Google Scholar] [CrossRef] [PubMed]
| Variables Data | N (%) | p-Value | |
|---|---|---|---|
| No MetS (n = 4673) | MetS (n = 578) | ||
| Male | 2191 (46.9) | 241 (41.7) | 0.018 * |
| Female | 2482 (53.1) | 337 (58.3) | |
| Age (arithmetic mean ± SE, year) | 49.87 ± 0.22 | 61.59 ± 0.50 | <0.001 * |
| Age group (year) | |||
| 20–29 | 439 (9.4) | 9 (1.6) | <0.001 * |
| 30–39 | 860 (18.4) | 23 (4.0) | |
| 40–49 | 974 (20.8) | 50 (8.7) | |
| 50–59 | 1038 (22.2) | 133 (23.0) | |
| 60–69 | 855 (18.3) | 197 (34.1) | |
| 70+ | 507 (10.8) | 166 (28.7) | |
| Body Mass Index (BMI) (arithmetic mean ± SE, kg/m2) | 24.05 ± 0.05 | 27.02 ± 0.16 | <0.001 * |
| Obesity | |||
| Normal (BMI < 30) | 4516 (96.6) | 436 (75.4) | <0.001 * |
| Obese (BMI ≥ 30) | 157 (3.4) | 142 (24.6) | |
| Smoking Status | |||
| Non-smoker | 2950 (63.1) | 384 (66.4) | 0.100 |
| Past-smoker | 815 (17.4) | 103 (17.8) | |
| Current smoker | 908 (19.4) | 91 (15.7) | |
| Drinking Status | |||
| Non-drinker | 1468 (31.4) | 270 (46.7) | <0.001 * |
| Past-drinker | 293 (6.3) | 57 (9.9) | |
| Current drinker | 2912 (62.3) | 251 (43.4) | |
| Education level | |||
| <High school | 1419 (30.4) | 346 (59.9) | <0.001 * |
| High school | 1464 (31.3) | 152 (26.3) | |
| College and more | 1790 (38.3) | 80 (13.8) | |
| Regular exercise | |||
| Yes | 1697 (36.3) | 211 (36.5) | 0.929 |
| No | 2976 (63.7) | 367 (63.5) | |
| Marital status | |||
| Single | 529 (11.3) | 13 (2.2) | <0.001 * |
| Married | 3740 (80.0) | 453 (78.4) | |
| Divorce/Separation | 404 (8.6) | 112 (19.4) | |
| Income level1 | |||
| Good | 41 (0.9) | 7 (1.2) | <0.001 * |
| Average | 3424 (73.3) | 329 (56.9) | |
| Bad | 1208 (25.9) | 242 (41.9) | |
| Alanine aminotransferase (arithmetic mean ± SE, U/L) | 23.8 ± 0.3 | 28.0 ± 0.8 | <0.001 * |
| Aspartate aminotransferase (arithmetic mean ± SE, U/L) | 25.1 ± 0.2 | 26.8 ± 0.5 | <0.001 * |
| Variables | No MetS (n = 4673) | MetS (n = 578) | p-Value |
|---|---|---|---|
| Urinary heavy metal (geometric mean ± SE) | |||
| Urinary cadmium (μg/dL) | −0.591 ± 0.688 | −0.320 ± 0.635 | <0.001 * |
| Blood heavy metal (geometric mean ± SE) | |||
| Blood lead (μg/dL) | 0.713 ± 0.482 | 0.759 ± 0.487 | 0.031 * |
| Blood mercury (μg/dL) | 1.180 ±0.640 | 1.165 ± 0.664 | 0.594 |
| Urinary VOCs metabolite (geometric mean ± SE) | |||
| Hippuric acid (g/dL) | −1.610 ± 0.016 | −1.560 ± 0.493 | 0.321 |
| Muconic acid (μg/dL) | 4.400 ± 0.114 | 4.479 ± 0.032 | 0.021 * |
| Phenylglyoxylic acid (mg/dL) | −1.508 ± 0.123 | −1.380 ± 0.331 | 0.001 * |
| Mandelic acid (mg/dL) | −1.563 ± 0.011 | −1.455 ± 0.029 | 0.001 * |
| Sum of urinary MHA isomer (geometric mean ± SE) | −1.183 ± 0.014 | −1.260 ± 0.039 | 0.077 |
| Urinary phthalate metabolite (geometric mean ± SE) | |||
| MEHHP (μg/dL) | 3.275 ± 0.010 | 3.485 ± 0.026 | <0.001 * |
| MEOHP (μg/dL) | 2.912 ± 0.010 | 3.129 ± 0.029 | <0.001 * |
| MECPP (μg/dL) | 3.380 ± 0.009 | 3.570 ± 0.026 | <0.001 * |
| MnBP (μg/dL) | 3.570 ± 0.010 | 3.700 ± 0.031 | <0.001 * |
| MBzP (μg/dL) | 1.388 ± 0.015 | 1.552 ± 0.040 | <0.001 * |
| Urinary bisphenol A (geometric mean ± SE) (μg/dL) | 0.038 ± 0.016 | 0.225 ± 0.056 | 0.001 * |
| Outcome Variable | Model 1 1 | Model 2 2 | Model 3 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Metabolic syndrome | ||||||
| Urinary cadmium | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.475 (1.102–1.973) | 0.009 * | 0.908 (0.668–1.233) | 0.536 | 0.914 (0.670–1.246) | 0.569 |
| Quartile 3 | 1.967 (1.489–2.600) | <0.001 * | 0.930 (0.689–1.255) | 0.633 | 0.929 (0.685–1.259) | 0.633 |
| Quartile 4 | 3.016 (2.314–3.931) | <0.001 * | 1.098 (0.815–1.479) | 0.539 | 1.094 (0.809–1.480) | 0.558 |
| Blood lead | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.159 (0.899–1.494) | 0.256 | 0.951 (0.728–1.244) | 0.716 | 0.941 (0.717–1.236) | 0.663 |
| Quartile 3 | 1.358 (1.059–1.742) | 0.016 * | 0.974 (0.745–1.272) | 0.844 | 0.999 (0.763–1.309) | 0.994 |
| Quartile 4 | 1.262 (0.982–1.623) | 0.069 | 0.794 (0.601–1.049) | 0.105 | 0.859 (0.648–1.138) | 0.289 |
| Blood mercury | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 0.776 (0.549–1.097) | 0.152 | 0.883 (0.614–1.270) | 0.503 | 0.844 (0.584–1.218) | 0.364 |
| Quartile 3 | 0.838 (0.604–1.161) | 0.288 | 1.019 (0.722–1.439) | 0.913 | 0.948 (0.669–1.344) | 0.765 |
| Quartile 4 | 0.863 (0.656–1.136) | 0.295 | 1.078 (0.801–1.449) | 0.621 | 0.990 (0.733–1.337) | 0.947 |
| Urinary muconic acid | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.133 (0.880–1.460) | 0.333 | 1.074 (0.826–1.398) | 0.593 | 1.066 (0.817–1.390) | 0.636 |
| Quartile 3 | 1.298 (1.013–1.662) | 0.039 * | 1.330 (1.028–1.721) | 0.030 * | 1.311 (1.011–1.700) | 0.041 * |
| Quartile 4 | 1.234 (0.962–1.584) | 0.098 | 1.453 (1.118–1.888) | 0.005 * | 1.393 (1.069–1.816) | 0.014 * |
| Urinary phenylgloxylic acid | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.096 (0.842–1.426) | 0.498 | 1.133 (0.861–1.490) | 0.373 | 1.074 (0.814–1.417) | 0.615 |
| Quartile 3 | 1.469 (1.144–1.887) | 0.003 * | 1.298 (1.000–1.684) | 0.050 * | 1.236 (0.950–1.609) | 0.115 |
| Quartile 4 | 1.510 (1.177–1.938) | 0.001 * | 1.204 (0.930–1.560) | 0.159 | 1.171 (0.902–1.520) | 0.235 |
| Urinary mandelic acid | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 0.960 (0.737–1.249) | 0.759 | 0.862 (0.656–1.134) | 0.290 | 0.819 (0.621–1.081) | 0.158 |
| Quartile 3 | 1.303 (1.016–1.672) | 0.037 * | 1.084 (0.836–1.406) | 0.544 | 1.022 (0.785–1.329) | 0.874 |
| Quartile 4 | 1.481 (1.160–1.889) | 0.002 * | 1.131 (0.878–1.458) | 0.341 | 1.097 (0.849–1.418) | 0.479 |
| Urinary MEHHP | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.746 (1.317–2.315) | <0.001 * | 1.326 (0.989–1.778) | 0.059 | 1.345 (1.001–1.808) | 0.050 * |
| Quartile 3 | 1.862 (1.408–2.462) | <0.001 * | 1.163 (0.866–1.561) | 0.316 | 1.151 (0.854–1.550) | 0.356 |
| Quartile 4 | 2.709 (2.075–3.537) | <0.001 * | 1.339 (1.003–1.788) | 0.048 * | 1.334 (0.996–1.787) | 0.054 |
| Urinary MEOHP | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.632 (1.231–2.164) | 0.001 * | 1.185 (0.883–1.589) | 0.258 | 1.193 (0.886–1.607) | 0.245 |
| Quartile 3 | 1.966 (1.494–2.587) | <0.001 * | 1.171 (0.875–1.567) | 0.289 | 1.213 (0.902–1.631) | 0.201 |
| Quartile 4 | 2.534 (1.942–3.305) | <0.001 * | 1.156 (0.862–1.550) | 0.333 | 1.181 (0.877–1.591) | 0.273 |
| Urinary MECCP | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.691 (1.277–2.238) | <0.001 * | 1.260 (0.940–1.688) | 0.122 | 1.280 (0.952–1.721) | 0.102 |
| Quartile 3 | 1.870 (1.418–2.465) | <0.001 * | 1.132 (0.844–1.518) | 0.407 | 1.122 (0.834–1.509) | 0.447 |
| Quartile 4 | 2.579 (1.978–3.362) | <0.001 * | 1.204 (0.898–1.616) | 0.215 | 1.176 (0.873–1.584) | 0.285 |
| Urinary MnBP | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.190 (0.918–1.542) | 0.189 | 1.020 (0.778–1.337) | 0.886 | 1.052 (0.800–1.384) | 0.716 |
| Quartile 3 | 1.377 (1.069–1.774) | 0.013 * | 0.992 (0.759–1.297) | 0.953 | 1.046 (0.798–1.373) | 0.744 |
| Quartile 4 | 1.490 (1.160–1.912) | 0.002 * | 0.860 (0.658–1.124) | 0.268 | 0.897 (0.684–1.176) | 0.430 |
| Urinary MBzP | ||||||
| Quartile 1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| Quartile 2 | 1.318 (1.015–1.711) | 0.038 * | 1.219 (0.930–1.598) | 0.152 | 1.247 (0.948–1.640) | 0.114 |
| Quartile 3 | 1.453 (1.124–1.878) | 0.004 * | 1.097 (0.839–1.434) | 0.5 | 1.100 (0.839–1.444) | 0.490 |
| Quartile 4 | 1.615 (1.254–2.078) | <0.001 * | 1.070 (0.821–1.396) | 0.616 | 1.090 (0.834–1.426) | 0.527 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, Y.H.; Ock, J.W.; Kim, Y.-J.; Kim, Y.; Kim, S.Y.; Kang, D. Association between Heavy Metals, Bisphenol A, Volatile Organic Compounds and Phthalates and Metabolic Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 671. https://doi.org/10.3390/ijerph16040671
Shim YH, Ock JW, Kim Y-J, Kim Y, Kim SY, Kang D. Association between Heavy Metals, Bisphenol A, Volatile Organic Compounds and Phthalates and Metabolic Syndrome. International Journal of Environmental Research and Public Health. 2019; 16(4):671. https://doi.org/10.3390/ijerph16040671
Chicago/Turabian StyleShim, Yun Hwa, Jung Won Ock, Yoon-Ji Kim, Youngki Kim, Se Yeong Kim, and Dongmug Kang. 2019. "Association between Heavy Metals, Bisphenol A, Volatile Organic Compounds and Phthalates and Metabolic Syndrome" International Journal of Environmental Research and Public Health 16, no. 4: 671. https://doi.org/10.3390/ijerph16040671
APA StyleShim, Y. H., Ock, J. W., Kim, Y. -J., Kim, Y., Kim, S. Y., & Kang, D. (2019). Association between Heavy Metals, Bisphenol A, Volatile Organic Compounds and Phthalates and Metabolic Syndrome. International Journal of Environmental Research and Public Health, 16(4), 671. https://doi.org/10.3390/ijerph16040671