Long-Term Heavy Metal Retention by Mangroves and Effect on Its Growth: A Field Inventory and Scenario Simulation
Abstract
:1. Introduction
- (i)
- Review and re-evaluate HMs contamination degrees in mangrove soil;
- (ii)
- assess the effects of the long-term HM pollution on the growth of R. apiculata in the interactions with the effects of the natural factors soil salinity, ground elevation and tree density; and
- (iii)
- perform scenario simulations of mangrove dynamics under different levels of HM pollution and other natural stressors.
2. Materials and Methods
2.1. The Can Gio Mangrove Forest
2.2. Sampling Positions, Samples Preparation, and Analysis
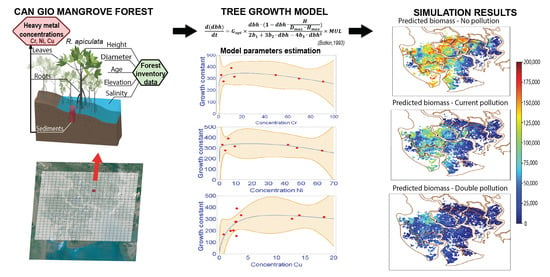
2.3. Tree Growth Model
2.3.1. Tree Growth Equation
2.3.2. Calculation of Tree Growth Rate G
2.4. Data Analysis and Model Parameters Estimation
2.4.1. Data Analysis
2.4.2. Model Parameter Estimation
2.5. Scenarios Setting
- (i)
- The baseline scenario (SC0) which assumed the unpolluted environmental condition. This scenario projects how the system may have developed within a four-decade period (1978–2020) under ideal conditions of no HM pollution.
- (ii)
- Scenario based on actual chromium pollution (SC0P1) in the area in accordance with our observational data.
- (iii)
- Worst case scenario assuming a twofold chromium load (SC0P2).
3. Results and Discussion
3.1. HMs Pollution in the Soil and Potential of HMs Retention by R. apiculata
3.1.1. Soil HMs Pollution in the Area
3.1.2. Potential of the HMs Retention by the Mangrove Species R. apiculata
3.2. Growth of R. apiculata in Different Environmental Conditions
3.2.1. Tree Growth Rate (G) and Influencing Factors
3.2.2. Tree Growth Rate (G) in Polluted Condition
3.3. Simulation Results
3.3.1. Model Validation
3.3.2. Scenarios Simulation Results
3.4. Toward a Balancing Management Approach on Using Mangroves to Clean Up Polluted Environment and to Protect the Mangrove Ecosystem
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- MacFarlane, G.R.; Koller, C.E.; Blomberg, S.P. Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere 2007, 69, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hossain, M.B.; Babu, S.M.O.F.; Rahman, M.; Ahmed, A.S.S.; Jolly, Y.N.; Choudhury, T.R.; Begum, B.A.; Kabir, J.; Akter, S. Source of metal contamination in sediment, their ecological risk, and phytoremediation ability of the studied mangrove plants in ship breaking area, Bangladesh. Mar. Pollut. Bull. 2019, 141, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.J.; Otte, M.L. Wetland plant effects on the biogeochemistry of metals beyond the rhizosphere. Biol. Environ. Proc. R. Ir. Acad. 1999, 99B, 3–10. [Google Scholar]
- Kamaruzzaman, B.Y.; Ong, M.C.; Jalal, K.C.A.; Shahbudin, S.; Nor, O.M. Accumulation of lead and copper in Rhizophora apiculata from Setiu mangrove forest, Terengganu, Malaysia. J. Environ. Biol. 2009, 30, 821. [Google Scholar] [PubMed]
- MacFarlane, G.R.; Pulkownik, A.; Burchett, M.D. Accumulation and distribution of heavy metals in the grey mangrove, Avicennia marina (Forsk.)Vierh.: Biological indication potential. Environ. Pollut. 2003, 123, 139–151. [Google Scholar] [CrossRef]
- Nazli, M.F.; Hashim, N.R. Heavy Metal Concentrations in an Important Mangrove Species, Sonneratia caseolaris, in Peninsular Malaysia. Environ. Asia 2010, 3, 50–55. [Google Scholar] [CrossRef]
- Chowdhury, R.; Favas, P.J.C.; Jonathan, M.P.; Venkatachalam, P.; Raja, P.; Sarkar, S.K. Bioremoval of trace metals from rhizosediment by mangrove plants in Indian Sundarban Wetland. Mar. Pollut. Bull. 2017, 124, 1078–1088. [Google Scholar] [CrossRef]
- Fengzhong, Z.; Liyu, H.; Wenjiao, Z. A primary study on adsorption of certain heavy metals on the litter leaf detritus of some mangrove species. J. Xiamen Univ. (Nat. Sci.) 1998, 37, 137–141. [Google Scholar]
- Wen-jiao, Z.; Xiao-yong, C.; Peng, L. Accumulation and biological cycling of heavy metal elements in Rhizophora stylosa mangroves in Yingluo Bay, China. Mar. Ecol. Prog. Ser. 1997, 159, 293–301. [Google Scholar]
- Titah, H.; Pratikno, H. Chromium Accumulation by Avicennia alba Growing at Ecotourism Mangrove Forest in Surabaya, Indonesia. J. Ecol. Eng. 2020, 21, 222–227. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Xu, L. Mechanisms of copper toxicity and resistance of plants. Ecol. Environ. 2003, 3, 336–341. [Google Scholar]
- Sandilyan, S.; Kathiresan, K. Decline of mangroves—A threat of heavy metal poisoning in Asia. Ocean Coast. Manag. 2014, 102, 161–168. [Google Scholar] [CrossRef]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Yan, Z.; Tam, N.F.Y. Effects of lead stress on anti-oxidative enzymes and stress-related hormones in seedlings of Excoecaria agallocha Linn. Plant Soil 2013, 367, 327–338. [Google Scholar] [CrossRef]
- Guo, X.Y.; Yan, C.L.; Ye, B.B. Effect of Cd–Zn Combined stress on contents of osmotic substances in Kandelia candel (L.) druce seedlings. Chin. J. Appl. Environ. Biol. 2009, 15, 308–312. [Google Scholar] [CrossRef]
- Li, P.; Zhao, C.; Zhang, Y.; Wang, X.; Wang, X.; Wang, J.; Wang, F.; Bi, Y. Calcium alleviates cadmium-induced inhibition on root growth by maintaining auxin homeostasis in Arabidopsis seedlings. Protoplasma 2016, 253, 185–200. [Google Scholar] [CrossRef]
- Lugo, A.E.; Snedaker, S.C. The Ecology of Mangroves. Annu. Rev. Ecol. Syst. 1974, 5, 39–64. [Google Scholar] [CrossRef]
- Thom, B.G. Mangrove ecology—A geomorphological perspective. In Mangrove Ecosystems in Australia; Clough, B.F., Ed.; Australian National University Press: Canberra, Australia, 1982; pp. 3–17. [Google Scholar]
- Feller, I.C.; Lovelock, C.E.; Berger, U.; McKee, K.L.; Joye, S.B.; Ball, M.C. Biocomplexity in Mangrove Ecosystems. Annu. Rev. Mar. Sci. 2009, 2, 395–417. [Google Scholar] [CrossRef] [Green Version]
- Twilley, R.R.; Rivera-Monroy, V.H. Developing performance measures of mangrove wetlands using simulation models of hydrology, nutrient biogeochemistry, and community dynamics. J. Coast. Res. 2005, 40, 79–93. [Google Scholar]
- Ewel, K.; Bourgeois, J.; Cole, T.; Zheng, S. Variation in environmental characteristics and vegetation in high-rainfall mangrove forests, Kosrae, Micronesia. Glob. Ecol. Biogeogr. Lett. 1998, 7, 49–56. [Google Scholar] [CrossRef]
- Chen, R.; Twilley, R.R. A gap dynamic model of mangrove forest development along gradients of soil salinity and nutrient resources. J. Ecol. 1998, 86, 37–51. [Google Scholar] [CrossRef]
- Grueters, U.; Ibrahim, M.R.; Satyanarayana, B.; Dahdouh-Guebas, F. Individual-based modeling of mangrove forest growth: MesoFON—Recent calibration and future direction. Estuar. Coast. Shelf Sci. 2019, 227, 106302. [Google Scholar] [CrossRef] [Green Version]
- Berger, U.; Hildenbrandt, H. A new approach to spatially explicit modelling of forest dynamics: Spacing, ageing and neighbourhood competition of mangrove trees. Ecol. Model. 2000, 132, 287–302. [Google Scholar] [CrossRef]
- Rideout, A.J.R.; Joshi, N.P.; Viergever, K.M.; Huxham, M.; Briers, R.A. Making predictions of mangrove deforestation: A comparison of two methods in Kenya. Glob. Chang. Biol. 2013, 19, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Ray, R.; González, A.G.; Pokrovsky, O.S.; Mavromatis, V.; Jana, T.K. Accumulation, transport and toxicity of arsenic in the Sundarbans mangrove, India. Geoderma 2019, 354, 113891. [Google Scholar] [CrossRef]
- Richter, O.; Nguyen, H.A.; Nguyen, K.L.; Nguyen, V.P.; Biester, H.; Schmidt, P. Phytoremediation by mangrove trees: Experimental studies and model development. Chem. Eng. J. 2016, 294, 389–399. [Google Scholar] [CrossRef]
- Nguyen, A.; Le, B.V.Q.; Richter, O. The Role of Mangroves in the Retention of Heavy Metal (Chromium): A Simulation Study in the Thi Vai River Catchment, Vietnam. Int. J. Environ. Res. Public Health 2020, 17, 5823. [Google Scholar] [CrossRef]
- Cormier Salem, M.; Nguyen, T.; Ariadna, B.; Durand, J.-D.; Bettarel, Y.; Klein, J.; Hoang, H.; Panfili, J. The mangrove’s contribution to people: Interdisciplinary pilot study of the Can Gio Mangrove Biosphere Reserve in Viet Nam. Comptes Rendus Geosci. 2017, 349, 341–350. [Google Scholar] [CrossRef]
- Kuenzer, C.; Tuan, V.Q. Assessing the ecosystem services value of Can Gio Mangrove Biosphere Reserve: Combining earth-observation- and household-survey-based analyses. Appl. Geogr. 2013, 45, 167–184. [Google Scholar] [CrossRef]
- Costa-Boddeker, S.; Hoelzmann, P.; Thuyen, L.X.; Huy, H.D.; Nguyen, H.A.; Richter, O.; Schwalb, A. Ecological risk assessment of a coastal zone in Southern Vietnam: Spatial distribution and content of heavy metals in water and surface sediments of the Thi Vai Estuary and Can Gio Mangrove Forest. Mar. Pollut. Bull. 2017, 114, 1141–1151. [Google Scholar] [CrossRef]
- Costa-Boddeker, S.; Thuyen, L.X.; Hoelzmann, P.; de Stigter, H.C.; van Gaever, P.; Huy, H.D.; Schwalb, A. The hidden threat of heavy metal pollution in high sedimentation and highly dynamic environment: Assessment of metal accumulation rates in the Thi Vai Estuary, Southern Vietnam. Environ. Pollut. 2018, 242, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.H. A Model for Predicting Mangrove Forest Dynamics under Variable Environmental Conditions—A Case Study of the Estuary of Dongnai—Saigon River System, Vietnam; Technischen Universität Carolo-Wilhelmina: Braunschweig, Germany, 2011. [Google Scholar]
- Hang, H.T.; Anh, N.H. Study on using vegetation indicator in monitoring the change of soil features in Can Gio area with the help of remote sensing. J. Sci. Technol. Dev. Vietnam 2002, 5, 10–17. (In Vietnamese) [Google Scholar]
- Phan Nguyen, H. Effects of mangrove restoration and conservation on the biodiversity and environment in Can Gio District. In Proceedings of the Mangrove Management and Conservation Workshop, Okinawa, Japan, 26–30 March 2000; pp. 111–137. [Google Scholar]
- van Loon, A.F.; Dijksma, R.; van Mensvoort, M.E.F. Hydrological classification in mangrove areas: A case study in Can Gio, Vietnam. Aquat. Bot. 2007, 87, 80–82. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Richter, O.; Huynh, D.H.; Nguyen, K.L.; Kolb, M.; Nguyen, V.P.; Bao, T.T. Accumulation of contaminants in mangrove species Rhizophora apiculate along Thi Vai River in the South of Vietnam. In EWATEC-COAST: Technologies for Environmental and Water Protection of Coastal Regions in Vietnam: Contributions to 4th International Conference for Environment and Natural Resources—ICENR 2014; Meon, G., Pätsch, M., Phuoc, N.V., Quan, N.H., Eds.; Cuvillier Verlag: Göttingen, Germany, 2014. [Google Scholar]
- Costa-Böddeker, S.; Thuyên, L.X.; Hoelzmann, P.; de Stigter, H.C.; van Gaever, P.; Huy, H.Đ.; Smol, J.P.; Schwalb, A. Heavy metal pollution in a reforested mangrove ecosystem (Can Gio Biosphere Reserve, Southern Vietnam): Effects of natural and anthropogenic stressors over a thirty-year history. Sci. Total Environ. 2020, 716, 137035. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, K.; Nguyen, T.; Ricklefs, K. Sediment re-deposition in the mangrove environment of Can Gio, Saigon River estuary (Vietnam). J. Coast. Res. 2016, SPI 75, 138–142. [Google Scholar] [CrossRef] [Green Version]
- ISO. 12914:2012 Soil Quality—Microwave Assisted Extraction of the Aqua Regia Soluble Fraction for the Determination of Elements; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- ISO. 11466:1995 Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Botkin, D.B. Forest Dynamics: An Ecological Model; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego Páramo, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant Sci. Int. J. Exp. Plant Biol. 2013, 212, 15–25. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef]
- Sinex, S.A.; Wright, D.A. Distribution of trace metals in the sediments and biota of Chesapeake Bay. Mar. Pollut. Bull. 1988, 19, 425–431. [Google Scholar] [CrossRef]
- Yongming, H.; Peixuan, D.; Junji, C.; Posmentier, E.S. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ. 2006, 355, 176–186. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.L. Riverine Composition and Estuarine Geochemistry of Particulate Metals in China—Weathering Features, Anthropogenic Impact and Chemical Fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The geochemical evolution of the continental crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Feng, J.; Nie, X.-P. Recent environmental changes reflected by metals and biogenic elements in sediments from the Guishan Island, the Pearl River Estuary, China. Estuar. Coast. Shelf Sci. 2015, 164, 493–505. [Google Scholar] [CrossRef]
- Nam, V.N.; Thuy, N.S.; Thanh, C.V.; Ha, H.S.; Tra, V.L.; Hoan, H.D. Development of Allometric Relationship between Biomass and Growth Params to Build Up a Reference Table for Mangrove Silviculture Practice and Management; Technical Report; Department of Agriculture and Rural Development of Ho Chi Minh City: Ho Chi Minh City, Vietnam, December 2004; p. 74. (In Vietnamese)
- Peterson, G.; Cumming, G.; Carpenter, S. Scenario Planning: A Tool for Conservation in an Uncertain World. Conserv. Biol. 2003, 17, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.A.; Richter, O.; Nguyen, V.P. Multiphysics Modeling of Pollutant Uptake by Mangroves. In Modeling, Simulation and Optimization of Complex Processes HPSC 2015; Springer: Cham, Switzerland, 2017; pp. 127–138. [Google Scholar]
- Ong Che, R.G. Concentration of 7 Heavy Metals in Sediments and Mangrove Root Samples from Mai Po, Hong Kong. Mar. Pollut. Bull. 1999, 39, 269–279. [Google Scholar] [CrossRef]
- Pratikno, H.; Titah, H.S.; Handayanu, H.; Harnani, B.R.D. The ability of Avicennia marina (Api-api putih) to Uptake Heavy Metal of Chromium at Wonorejo Coastal in Surabaya. In Proceedings of the International Conference on Coastal and Delta Areas, Semarang, Indonesia, 26 September 2017; pp. 164–169. [Google Scholar]
- Alharbi, O.M.L.; Khattab, R.A.; Ali, I.; Binnaser, Y.S.; Aqeel, A. Assessment of heavy metals contamination in the sediments and mangroves (Avicennia marina) at Yanbu coast, Red Sea, Saudi Arabia. Mar. Pollut. Bull. 2019, 149, 110669. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, X.; Xu, Y.; Zhang, Q.; Li, X. Accumulation and Tolerance of Mangroves to Heavy Metals: A Review. Curr. Pollut. Rep. 2017, 3, 302–317. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, Y.S. Retention of nutrients and heavy metals in mangrove sediment receiving wastewater of different strengths. Environ. Technol. 1993, 14, 719–729. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Carvalho, C.E.V.; Tanizaki, K.F.; Ovalle, A.R.C.; Rezende, C.E. The Biogeochemistry and Trace Metals Distribution of Mangrove Rhizospheres. Biotropica 1993, 25, 252–257. [Google Scholar] [CrossRef]
- Carlos Augusto, R.S.; Lacerda, L.D.; Rezende, C.E. Metals Reservoir in a Red Mangrove Forest. Biotropica 1990, 22, 339–345. [Google Scholar] [CrossRef]
- Stigliani, W.M. Global Perspectives and Risk Assessment. In Biogeodynamics of Pollutants in Soils and Sediments: Risk Assessment of Delayed and Non-Linear Responses; Salomons, W., Stigliani, W.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 331–343. [Google Scholar]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; Delaune, R.D.; Patrick, W.H. Flooding and saltwater intrusion: Potential effects on survival and productivity of wetland forests along the U.S. Gulf Coast. For. Ecol. Manag. 1990, 33–34, 287–301. [Google Scholar] [CrossRef]
- Ye, Y.; Tam, N.F.-Y.; Lu, C.-Y.; Wong, Y.-S. Effects of salinity on germination, seedling growth and physiology of three salt-secreting mangrove species. Aquat. Bot. 2005, 83, 193–205. [Google Scholar] [CrossRef]
- Reef, R.; Feller, I.; Lovelock, C. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; Trettin, C.C.; Frolking, S.; Birdsey, R.A. Mangrove carbon assessment tool: Model development and sensitivity analysis. Estuar. Coast. Shelf Sci. 2018, 208, 23–35. [Google Scholar] [CrossRef]
- Ball, M.C.; Pidsley, S.M. Growth Responses to Salinity in Relation to Distribution of Two Mangrove Species, Sonneratia alba and S. lanceolata, in Northern Australia. Funct. Ecol. 1995, 9, 77–85. [Google Scholar] [CrossRef]
- Takemura, T.; Hanagata, N.; Sugihara, K.; Baba, S.; Karube, I.; Dubinsky, Z. Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza. Aquat. Bot. 2000, 68, 15–28. [Google Scholar] [CrossRef]
- Suárez, N.; Medina, E. Salinity effect on plant growth and leaf demography of the mangrove, Avicennia germinans L. Trees 2005, 19, 722. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Nguyen, H.A.; Richter, O.; Pham, M.T.; Nguyen, V.P. Ecophysiological responses of young mangrove species Rhizophora apiculata (Blume) to different chromium contaminated environments. Sci. Total Environ. 2017, 574, 369–380. [Google Scholar] [CrossRef]
- Tri, N.H.; Hong, P.N.; Cuc, L.T. Can Gio Mangrove Biosphere Reserve; Cartographic Publishing House: Hanoi, Vietnam, 2000. [Google Scholar]
- Alongi, D.M. Present state and future of the world’s mangrove forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Ellison, A.M.; Farnsworth, E.J. Simulated sea level change alters anatomy, physiology, growth, and reproduction of red mangrove (Rhizophora mangle L.). Oecologia 1997, 112, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-E.; Liu, J.-L.; Ouyang, Y.; Liao, B.-W.; Zhao, B.-L. Physiological responses of mangrove Sonneratia apetala Buch-Ham plant to wastewater nutrients and heavy metals. Int. J. Phytoremediat. 2011, 13, 456–464. [Google Scholar] [CrossRef]
- MacFarlane, G.R.; Burchett, M.D. Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar. Environ. Res. 2002, 54, 65–84. [Google Scholar] [CrossRef]
- Chawla, A.; Patel, K. Effect of Manganese and Copper stress on a leaf biochemical parameter of a mangrove Avicennia marina (Forsk.) Vierh. Eur. J. Biomed. Pharm. Sci. 2018, 5, 250–253. [Google Scholar]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Merchant, S.S. The Elements of Plant Micronutrients. Plant Physiol. 2010, 154, 512. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.S.A.; Ashraf, M. Essential Roles and Hazardous Effects of Nickel in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; pp. 125–167. [Google Scholar]
- Vinh, T.V.; Marchand, C.; Linh, T.V.K.; Vinh, D.D.; Allenbach, M. Allometric models to estimate above-ground biomass and carbon stocks in Rhizophora apiculata tropical managed mangrove forests (Southern Viet Nam). For. Ecol. Manag. 2019, 434, 131–141. [Google Scholar] [CrossRef]
- Hoan, H.D. Determine on the capacity of carbon accumulation of Rhizophora apiculata Blume plantation forests in Can Gio Mangrove Biosphere Reserve, Ho Chi Minh City. Ph.D. Thesis, Vietnamese Academy of Forest Sciences, Hanoi, Vietnam, January 2019. [Google Scholar]
- Bakhtiyari, M.; Lee, S.Y.; Warnken, J. Seeing the forest as well as the trees: An expert opinion approach to identifying holistic condition indicators for mangrove ecosystems. Estuar. Coast. Shelf Sci. 2019, 222, 183–194. [Google Scholar] [CrossRef]
- Sutula, M.A.; Stein, E.D.; Collins, J.N.; Fetscher, A.E.; Clark, R. A Practical Guide for the Development of a Wetland Assessment Method: The California Experience1. JAWRA J. Am. Water Resour. Assoc. 2006, 42, 157–175. [Google Scholar] [CrossRef]
- Sheaves, M.; Brookes, J.; Coles, R.; Freckelton, M.; Groves, P.; Johnston, R.; Winberg, P. Repair and revitalisation of Australia’s tropical estuaries and coastal wetlands: Opportunities and constraints for the reinstatement of lost function and productivity. Mar. Policy 2014, 47, 23–38. [Google Scholar] [CrossRef]









| SOIL | ROOT | LEAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Cr | Ni | Cu | Cr | Ni | Cu | Cr | Ni | |||
| CG | Upstream | NP1 | 80.04 | 4.96 | 3.31 | 0.21 | 1.06 | 0.57 | 0.31 | 1.51 | 0.71 |
| DK4 | 70.82 | 3.76 | 2.72 | 0.60 | 0.70 | 0.45 | 0.72 | 0.37 | 0.12 | ||
| DK6 | 64.51 | 4.35 | 2.63 | 0.31 | 0.32 | 0.11 | 0.25 | 0.29 | 0.01 | ||
| DK7 | 65.65 | 4.44 | 3.08 | 0.54 | 0.26 | 0.17 | 0.39 | 0.40 | 0.09 | ||
| DK8 | 75.76 | 4.28 | 3.25 | 0.37 | 1.20 | 0.66 | 0.18 | 0.23 | 0.01 | ||
| Downstream | NP2 | 76.62 | 4.49 | 3.06 | 0.47 | 0.32 | 0.22 | 0.36 | 1.27 | 0.46 | |
| TV | Upstream | DN1 | 62.41 | 12.39 | 8.49 | 3.07 | 4.46 | 4.90 | 3.53 | 0.39 | 0.14 |
| DN2 | 134.03 | 19.84 | 11.35 | 8.17 | 11.69 | 8.54 | 2.12 | 0.49 | 0.01 | ||
| M5 | 60.40 | 21.14 | 12.01 | 5.55 | 2.19 | 2.19 | 4.03 | 1.04 | 0.27 | ||
| M3 | 79.46 | 13.62 | 8.82 | 4.88 | 1.52 | 1.30 | 3.40 | 2.05 | 1.82 | ||
| Downstream | M1 | 82.51 | 14.75 | 5.82 | 3.56 | 5.24 | 3.08 | 7.83 | 1.08 | 0.76 | |
| CG | max | 80.04 | 4.96 | 3.31 | 0.60 | 1.20 | 0.66 | 0.72 | 1.51 | 0.71 | |
| mean | 72.23 | 4.38 | 3.01 | 0.42 | 0.64 | 0.36 | 0.37 | 0.68 | 0.23 | ||
| min | 64.51 | 3.76 | 2.63 | 0.21 | 0.26 | 0.11 | 0.18 | 0.23 | 0.01 | ||
| std | 6.29 | 0.39 | 0.28 | 0.15 | 0.41 | 0.23 | 0.19 | 0.56 | 0.29 | ||
| TV | max | 134.03 | 21.14 | 12.01 | 8.17 | 11.69 | 8.54 | 7.83 | 2.05 | 1.82 | |
| mean | 83.76 | 16.35 | 9.30 | 5.05 | 5.02 | 4.00 | 4.18 | 1.01 | 0.60 | ||
| min | 82.51 | 14.75 | 5.82 | 3.56 | 5.24 | 3.08 | 7.83 | 1.08 | 0.76 | ||
| std | 29.79 | 3.90 | 2.48 | 2.01 | 4.03 | 2.87 | 2.16 | 0.66 | 0.74 | ||
| Position | Mean dbh (cm) | Mean Height (cm) | Density/Plot (10 × 10 sq·m) | Age (Year) | Porosity Salinity (ppt) | Mean Tree Growth Rate (G) | EF (Cu) | EF (Cr) | EF (Ni) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Can Gio | NP1 | Upstream | 18.62 | 1989.97 | 26 | 26 | 8.0 | 390.53 | 3.41 | 0.11 | 0.09 |
| DK4 | 10.46 | 1232.91 | 42 | 32 | 12.4 | 277.08 | 4.13 | 0.11 | 0.10 | ||
| DK6 | 22.29 | 2293.78 | 8 | 38 | 12.9 | 275.46 | 2.53 | 0.09 | 0.07 | ||
| DK7 | 15.06 | 1673.72 | 13 | 37 | 15.5 | 201.61 | 2.42 | 0.08 | 0.08 | ||
| DK8 | 19.71 | 2082.55 | 11 | 40 | 15.5 | 197.74 | 2.62 | 0.07 | 0.07 | ||
| NP2 | Downstream | 13.14 | 1501.13 | 16 | 39 | 18.2 | 166.91 | 3.29 | 0.10 | 0.09 | |
| Thi Vai | DN1 | Upstream | 16.13 | 1770.92 | 13 | 25 | 8.0 | 332.13 | 1.18 | 0.12 | 0.11 |
| DN2 | 16.16 | 1757.51 | 20 | 25 | 9.6 | 331.82 | 3.49 | 0.26 | 0.20 | ||
| M5 | 13.99 | 1574.53 | 11 | 31 | 11.3 | 317.36 | 1.83 | 0.32 | 0.24 | ||
| M3 | 11.97 | 1381.85 | 20 | 23 | 13.5 | 300.98 | 2.53 | 0.22 | 0.19 | ||
| M1 | Downstream | 12.45 | 1428.23 | 17 | 32 | 17.1 | 152.97 | 3.90 | 0.35 | 0.18 | |
| Location | Species | Part | Cr (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Reference |
|---|---|---|---|---|---|---|
| This study, Can Gio, Vietnam (2018) | Soil | Soil | 3.76–21.14 | 60.4–134.03 | 2.63–12.01 | This study |
| Rhizophora apiculata | Leaves | 0.23–2.05 | 0.18–7.83 | 0.005–1.82 | ||
| Roots | 0.26–11.7 | 0.21–8.2 | 0.11–8.54 | |||
| Can Gio, Vietnam (2013) | Soil | Soil | 86–241 | 19.7–32.7 | 56.6–247 | [37] |
| Rhizophora apiculata | Leaves | 1.48–23.6 | 2.77–6.41 | 1.4–25.7 | ||
| Roots | 2.14–90.6 | 0.746–18.3 | 1.79–62.9 | |||
| Can Gio, Vietnam (2002–2012) | Sediment core | Sediment | 107.70–208.80 | 26.74–82.32 | 56.25–82.99 | [32] Note: Thi Vai side, upstream |
| Can Gio, Vietnam (2002–2012) | Sediment core | Sediment | 51.6–82.5 | 11.5–38.3 | 24.7–46.5 | [32] Note: Thi Vai side, downstream |
| Can Gio, Vietnam (1977–2011) | Sediment core | Sediment | 27.1–71.5 | 7.1–27.0 | 11.7–56.3 | [38] Note: Can Gio side |
| Mai po, Hong Kong | Soil | Soil | 20–74.6 | 51.1–87.4 | 43.9–86.9 | [53] |
| Acanthus ilicifolius | Roots | 1.6–6.2 | 25.2–65.4 | 8–20.1 | ||
| Aegicerus corniculatum | Roots | 1.8–5.8 | 22.4–46.2 | 6–32.01 | ||
| Kandelia candel | Roots | 1.8–6.4 | 19.6–29.4 | 4–28.001 | ||
| Surabaya, Indonesia | Soil | Soil | 47–79.3 | [10,54] | ||
| Avicennia alba | Roots | 25.4–55.3 | ||||
| Avicennia marina | Roots | 28–92.25 | ||||
| Yanbu, Red Sea, Saudi Arabia | Soil | Soil | 14.9–289 | 17.2–217.2 | 27.3–241.8 | [55] |
| Avicennia marina | Leaves | 14.2–50.1 | 18.1–40.2 | 16.1–56.3 | ||
| Roots | 16.3–40.5 | 16.8–37.3 | 17.2–38.2 |
| Parameter | Value | ||||
|---|---|---|---|---|---|
| Density multiplier | 0.402741 | ||||
| 0.156347 | |||||
| (trees/100 m2) | 66.9241 | ||||
| Salinity multiplier | 0.21276 | ||||
| −0.4 | |||||
| (ppt) | 11.348 | ||||
| Elevation multiplier | 0.027 | ||||
| −0.96 | |||||
| 13.1 | |||||
| 1.48 | |||||
| 6.02 | |||||
| 0.11 | |||||
| Pollutant multiplier | Cu | Cr | Ni | ||
| 3.28 | 1.89 | 0.53 | |||
| 54.47 | 170.25 | 105.43 | |||
| 0.68 | 0.12 | 0.06 | |||
| 0.81 | 1.69 | 2.53 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.; Richter, O.; Le, B.V.Q.; Phuong, N.T.K.; Dinh, K.C. Long-Term Heavy Metal Retention by Mangroves and Effect on Its Growth: A Field Inventory and Scenario Simulation. Int. J. Environ. Res. Public Health 2020, 17, 9131. https://doi.org/10.3390/ijerph17239131
Nguyen A, Richter O, Le BVQ, Phuong NTK, Dinh KC. Long-Term Heavy Metal Retention by Mangroves and Effect on Its Growth: A Field Inventory and Scenario Simulation. International Journal of Environmental Research and Public Health. 2020; 17(23):9131. https://doi.org/10.3390/ijerph17239131
Chicago/Turabian StyleNguyen, Anh, Otto Richter, Bao V.Q. Le, Nguyen Thi Kim Phuong, and Kim Chi Dinh. 2020. "Long-Term Heavy Metal Retention by Mangroves and Effect on Its Growth: A Field Inventory and Scenario Simulation" International Journal of Environmental Research and Public Health 17, no. 23: 9131. https://doi.org/10.3390/ijerph17239131
APA StyleNguyen, A., Richter, O., Le, B. V. Q., Phuong, N. T. K., & Dinh, K. C. (2020). Long-Term Heavy Metal Retention by Mangroves and Effect on Its Growth: A Field Inventory and Scenario Simulation. International Journal of Environmental Research and Public Health, 17(23), 9131. https://doi.org/10.3390/ijerph17239131