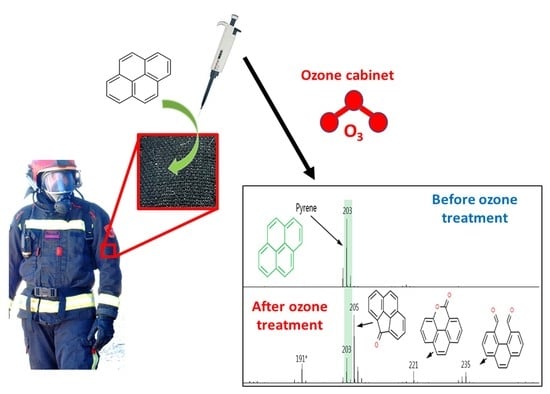
Evaluation of an Ozone Chamber as a Routine Method to Decontaminate Firefighters’ PPE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Homogeneous Experiments
2.2. Heterogeneous Experiments
2.3. Instrumentation
3. Results and Discussion
3.1. Homogeneous Experiments
3.2. Heterogeneous Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahy, R.F.; Petrillo, J.T.; Molis, J.L. National Fire Protection Association—Firefighter Fatalities in the US—2019; National Fire Protection Association: Quincy, MA, USA, 2020. [Google Scholar]
- Engelsman, M.; Toms, L.-M.L.; Banks, A.P.W.; Wang, X.; Mueller, J.F. Biomonitoring in firefighters for volatile organic compounds, semivolatile organic compounds, persistent organic pollutants, and metals: A systematic review. Environ. Res. 2020, 188, 109562. [Google Scholar] [CrossRef]
- World Health Organization (WHO) and International Agency for Research on Cancer (IARC). Painting, Firefighting, and Shiftwork/IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 1st ed.; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- LeMasters, G.K.; Genaidy, A.M.; Succop, P.; Deddens, J.; Sobeih, T.; Barriera-Viruet, H.; Dunning, K.; Lockey, J. Cancer Risk among Firefighters: A Review and Meta-analysis of 32 Studies. J. Occup. Environ. Med. 2006, 48, 1189–1202. [Google Scholar] [CrossRef]
- Daniels, R.D.; Kubale, T.L.; Yiin, J.H.; Dahm, M.M.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; Pinkerton, L.E. Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2014, 71, 388–397. [Google Scholar] [CrossRef]
- Daniels, R.D.; Bertke, S.; Dahm, M.M.; Yiin, J.H.; Kubale, T.L.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; et al. Exposure–response relationships for select cancer and non-cancer health outcomes in a cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2015, 72, 699–706. [Google Scholar] [CrossRef]
- Navarro, K.M.; Kleinman, M.T.; Mackay, C.E.; Reinhardt, T.E.; Balmes, J.R.; Broyles, G.A.; Ottmar, R.D.; Naher, L.P.; Domitrovich, J.W. Wildland firefighter smoke exposure and risk of lung cancer and cardiovascular disease mortality. Environ. Res. 2019, 173, 462–468. [Google Scholar] [CrossRef]
- Stec, A.A.; Dickens, K.E.; Salden, M.; Hewitt, F.E.; Watts, D.P.; Houldsworth, P.E.; Martin, F.L. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Sci. Rep. 2018, 8, 2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soteriades, E.S.; Kim, J.; Christophi, C.A.; Kales, S.N. Cancer Incidence and Mortality in Firefighters: A State-of-the-Art Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2019, 20, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, S.P.; Hao, W.M.; Baker, S. Chemical composition of wildland fire emissions. In Developments in Environmental Science; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Stec, A.A.; Hull, T.R. Fire Toxicity; Woodhead Publishing Limited: Cambridge, UK, 2010. [Google Scholar]
- Bralewska, K.; Rakowska, J. Concentrations of particulate matter and PM-bound polycyclic aromatic hydrocarbons released during combustion of various types of materials and possible toxicological potential of the emissions: The results of preliminary studies. Int. J. Environ. Res. Public Health 2020, 17, 3202. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, P.; Mc Namee, M.S.; Andersson, P.; Lönnermark, A. Polycyclic aromatic hydrocarbons (PAHs) quantified in large-scale fire experiments. Fire Technol. 2012, 48, 513–528. [Google Scholar] [CrossRef]
- Faboya, O.L.; Sojinu, S.O.; Oguntuase, B.J.; Sonibare, O.O. Impact of forest fires on polycyclic aromatic hydrocarbon concentrations and stable carbon isotope compositions in burnt soils from tropical forest, Nigeria. Sci. Afr. 2020, 8, e00331. [Google Scholar] [CrossRef]
- Horn, G.P.; Kerber, S.; Andrews, J.; Kesler, R.M.; Newman, H.; Stewart, J.W.; Fent, K.W.; Smith, D.L. Impact of Repeated Exposure and Cleaning on Protective Properties of Structural Firefighting Turnout Gear. Fire Technol. 2021, 57, 791–813. [Google Scholar] [CrossRef]
- Austin, C.C.; Dussault, G.; Ecobichon, D.J. Municipal firefighter exposure groups, time spent at fires and use of self-contained-breathing-apparatus. Am. J. Ind. Med. 2001, 40, 683–692. [Google Scholar] [CrossRef]
- Fent, K.W.; Alexander, B.; Roberts, J.; Robertson, S.; Toennis, C.; Sammons, D.; Bertke, S.; Kerber, S.; Smith, D.; Horn, G. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg. 2017, 14, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.M.; Logan, M.B. Structural Fire Fighting Ensembles: Accumulation and Off-gassing of Combustion Products. J. Occup. Environ. Hyg. 2015, 12, 376–383. [Google Scholar] [CrossRef]
- Fabian, T.Z.; Borgerson, J.L.; Gandhi, P.D.; Baxter, C.S.; Ross, C.S.; Lockey, J.E.; Dalton, J.M. Characterization of Firefighter Smoke Exposure. Fire Technol. 2014, 50, 993–1019. [Google Scholar] [CrossRef]
- Alharbi, B.H.; Pasha, M.J.; Al-Shamsi, M.A.S. Firefighter exposures to organic and inorganic gas emissions in emergency residential and industrial fires. Sci. Total Environ. 2021, 770, 145332. [Google Scholar] [CrossRef]
- Shinde, A.; Ormond, R.B. Development of a Headspace Sampling–Gas Chromatography–Mass Spectrometry Method for the Analysis of Fireground Contaminants on Firefighter Turnout Materials. ACS Chem. Health Saf. 2020, 27, 352–361. [Google Scholar] [CrossRef]
- Mayer, A.C.; Horn, G.P.; Fent, K.W.; Bertke, S.J.; Kerber, S.; Kesler, R.M.; Newman, H.; Smith, D.L. Impact of select PPE design elements and repeated laundering in firefighter protection from smoke exposure. J. Occup. Environ. Hyg. 2020, 17, 505–514. [Google Scholar] [CrossRef]
- Corbally, M.A.; Williams, M.R.; Chappell, J.N.; Sigman, M.E. Detecting Chemical Vapor Diffusion through Firefighter Turnout Gear. Int. J. Environ. Res. Public Health 2021, 18, 4833. [Google Scholar] [CrossRef]
- Banks, A.P.W.; Wang, X.; He, C.; Gallen, M.; Thomas, K.V.; Mueller, J.F. Off-Gassing of Semi-Volatile Organic Compounds from Fire-Fighters’ Uniforms in Private Vehicles—A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3030. [Google Scholar] [CrossRef]
- Natioanal Fire Protection Association. NFPA 1851—Standard on Selection, Care, and Maintenance of Protective Ensembles for Structural Fire Fighting and Proximity Fire Fighting; Natioanal Fire Protection Association: Orlando, FL, USA, 2020. [Google Scholar]
- Banks, A.P.W.; Wang, X.; Engelsman, M.; He, C.; Osorio, A.F.; Mueller, J.F. Assessing decontamination and laundering processes for the removal of polycyclic aromatic hydrocarbons and flame retardants from firefighting uniforms. Environ. Res. 2021, 194, 110616. [Google Scholar] [CrossRef]
- Anderson, D.A.; Harrison, T.R.; Yang, F.; Wendorf Muhamad, J.; Morgan, S.E. Firefighter perceptions of cancer risk: Results of a qualitative study. Am. J. Ind. Med. 2017, 60, 644–650. [Google Scholar] [CrossRef]
- Macy, G.B.; Hwang, J.; Taylor, R.; Golla, V.; Cann, C.; Gates, B. Examining Behaviors Related to Retirement, Cleaning, and Storage of Turnout Gear Among Rural Firefighters. Workplace Health Saf. 2020, 68, 129–138. [Google Scholar] [CrossRef]
- Broséus, R.; Vincent, S.; Aboulfadl, K.; Daneshvar, A.; Sauvé, S.; Barbeau, B.; Prévost, M. Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res. 2009, 43, 4707–4717. [Google Scholar] [CrossRef]
- Shezi, S.; Samukelo Magwaza, L.; Mditshwa, A.; Zeray Tesfay, S. Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Sci. Hortic. 2020, 269, 109397. [Google Scholar] [CrossRef]
- Breidablik, H.J.; Lysebo, D.E.; Johannessen, L.; Skare, Å.; Andersen, J.R.; Kleiven, O.T. Ozonized water as an alternative to alcohol-based hand disinfection. J. Hosp. Infect. 2019, 102, 419–424. [Google Scholar] [CrossRef]
- Fisher, T.J.; Dussault, P.H. Alkene ozonolysis. Tetrahedron 2017, 73, 4233–4258. [Google Scholar] [CrossRef]
- Aguilar, C.M.; Rodríguez, J.L.; Chairez, I.; Tiznado, H.; Poznyak, T. Naphthalene degradation by catalytic ozonation based on nickel oxide: Study of the ethanol as cosolvent. Environ. Sci. Pollut. Res. 2017, 24, 25550–25560. [Google Scholar] [CrossRef]
- Lundstedt, A.; Webb, M.J.; Grennberg, H. Ozonolysis of polycyclic aromatic hydrocarbons in participating solvents. RSC Adv. 2017, 7, 6152–6159. [Google Scholar] [CrossRef] [Green Version]
- Yerushalmi, L.; Nefil, S.; Hausler, R.; Guiot, S.R. Removal of Pyrene and Benzo(a)Pyrene from Contaminated Water by Sequential and Simultaneous Ozonation and Biotreatment. Water Environ. Res. 2006, 78, 2286–2292. [Google Scholar] [CrossRef]
- Turhan, K.; Uzman, S. Removal of phenol from water using ozone. Desalination 2008, 229, 257–263. [Google Scholar] [CrossRef]
- Yuan, R.; Zhou, B.; Ma, L. Removal of toluene from water by photocatalytic oxidation with activated carbon supported Fe3+-doped TiO2 nanotubes. Water Sci. Technol. 2014, 70, 642–648. [Google Scholar] [CrossRef]
- Cramer, B.; Humpf, H.-U. Applications of HPLC-MS techniques for the analysis of chemical contaminants and residues in food. In Chemical Contaminants and Residues in Food; Elsevier: Amsterdam, The Netherlands, 2012; pp. 62–78. [Google Scholar]
- Miet, K.; Le Menach, K.; Flaud, P.M.; Budzinski, H.; Villenave, E. Heterogeneous reactions of ozone with pyrene, 1-hydroxypyrene and 1-nitropyrene adsorbed on particles. Atmos. Environ. 2009, 43, 3699–3707. [Google Scholar] [CrossRef]
- Yao, J.-J.; Huang, Z.-H.; Masten, S.J. The ozonation of benz[a]anthracene: Pathway and product identification. Water Res. 1998, 32, 3235–3244. [Google Scholar] [CrossRef]
- Cochran, R.E.; Jeong, H.; Haddadi, S.; Fisseha Derseh, R.; Gowan, A.; Beránek, J.; Kubátová, A. Identification of products formed during the heterogeneous nitration and ozonation of polycyclic aromatic hydrocarbons. Atmos. Environ. 2016, 128, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Cochran, R.E.; Smoliakova, I.P.; Kubátová, A. Detection of nitrated and oxygenated polycyclic aromatic hydrocarbons using atmospheric pressure chemical ionization high resolution mass spectrometry. Int. J. Mass Spectrom. 2016, 397–398, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Hong, P.K.A.; Wavrek, D.A. Chemical-Biological Treatment of Pyrene. Water Res. 2000, 34, 1157–1172. [Google Scholar] [CrossRef]
- Choi, Y.-I.; Hong, A. Ozonation of polycyclic aromatic hydrocarbon in hexane and water: Identification of intermediates and pathway. Korean J. Chem. Eng. 2007, 24, 1003–1008. [Google Scholar] [CrossRef]
- Cajthaml, T.; Möder, M.; Kačer, P.; Šašek, V.; Popp, P. Study of fungal degradation products of polycyclic aromatic hydrocarbons using gas chromatography with ion trap mass spectrometry detection. J. Chromatogr. A 2002, 974, 213–222. [Google Scholar] [CrossRef]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, I.B.; Öberg, L.; Haglund, P.; Tysklind, M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. AMBIO J. Hum. Environ. 2007, 36, 475–485. [Google Scholar] [CrossRef]
- Halasinski, T.M.; Salama, F.; Allamandola, L.J. Investigation of the Ultraviolet, Visible, and Near-Infrared Absorption Spectra of Hydrogenated Polycyclic Aromatic Hydrocarbons and Their Cations. Astrophys. J. 2005, 628, 555–566. [Google Scholar] [CrossRef] [Green Version]
- Lindon, J.C.; Tranter, G.E.; Koppenaal, D. Encyclopedia of Spectroscopy and Spectrometry; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-0-12-803224-4. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucena, M.A.d.M.; Zapata, F.; Mauricio, F.G.M.; Ortega-Ojeda, F.E.; Quintanilla-López, M.G.; Weber, I.T.; Montalvo, G. Evaluation of an Ozone Chamber as a Routine Method to Decontaminate Firefighters’ PPE. Int. J. Environ. Res. Public Health 2021, 18, 10587. https://doi.org/10.3390/ijerph182010587
Lucena MAdM, Zapata F, Mauricio FGM, Ortega-Ojeda FE, Quintanilla-López MG, Weber IT, Montalvo G. Evaluation of an Ozone Chamber as a Routine Method to Decontaminate Firefighters’ PPE. International Journal of Environmental Research and Public Health. 2021; 18(20):10587. https://doi.org/10.3390/ijerph182010587
Chicago/Turabian StyleLucena, Marcella A. de Melo, Félix Zapata, Filipe Gabriel M. Mauricio, Fernando E. Ortega-Ojeda, M. Gloria Quintanilla-López, Ingrid Távora Weber, and Gemma Montalvo. 2021. "Evaluation of an Ozone Chamber as a Routine Method to Decontaminate Firefighters’ PPE" International Journal of Environmental Research and Public Health 18, no. 20: 10587. https://doi.org/10.3390/ijerph182010587
APA StyleLucena, M. A. d. M., Zapata, F., Mauricio, F. G. M., Ortega-Ojeda, F. E., Quintanilla-López, M. G., Weber, I. T., & Montalvo, G. (2021). Evaluation of an Ozone Chamber as a Routine Method to Decontaminate Firefighters’ PPE. International Journal of Environmental Research and Public Health, 18(20), 10587. https://doi.org/10.3390/ijerph182010587