Effect of Water-Pipe Smoking on the Normal Development of Zebrafish
Abstract
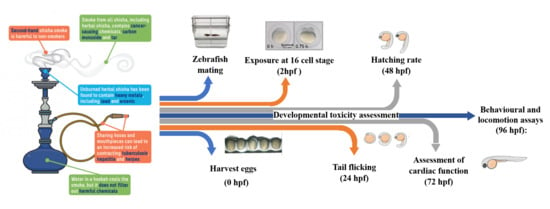
:1. Introduction
2. Materials and Methods
2.1. Smoking Machine Protocol and Water Pipe Preparation
2.2. Zebrafish Maintenance and Microscopy
2.3. Developmental Toxicity
2.4. Behavioural and Locomotion Assays
2.5. Cardiac Toxicity: Assessment of Cardiac Function
Live Imaging of Zebrafish
2.6. Cardiac Failure Markers Expression
2.7. Detection of Apoptotic Cells Using Acridine Orange (AO)
2.8. Statistical Analysis
3. Results
3.1. Developmental Toxicity
3.1.1. Survival Assay (24 hpf)
3.1.2. Hatching Rate (48 hpf)
3.1.3. Tail Flicking Assay (24 hpf)
3.2. Behavioural and Locomotion Assays (96 hpf)
3.3. Cardiac Toxicity: Assessment of Cardiac Function
Live Imaging of Zebrafish (72 hpf)
3.4. Cardiac Failure Markers Expression (72 hpf)
3.5. Acridine Orange Stainig for Apoptosis (72 hpf)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO. Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 1 December 2019).
- Marques, P.; Piqueras, L.; Sanz, M.-J. An Updated Overview of E-Cigarette Impact on Human Health. Respir. Res. 2021, 22, 1–14. [Google Scholar] [CrossRef]
- Onor, I.O.; Stirling, D.L.; Williams, S.R.; Bediako, D.; Borghol, A.; Harris, M.B.; Darensburg, T.B.; Clay, S.D.; Okpechi, S.C.; Sarpong, D.F. Clinical effects of cigarette smoking: Epidemiologic impact and review of pharmacotherapy options. Int. J. Environ. Res. Public Health 2017, 14, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maziak, W.; Taleb, Z.B.; Bahelah, R.; Islam, F.; Jaber, R.; Auf, R.; Salloum, R.G. The global epidemiology of waterpipe smoking. Tob. Control 2015, 24, i3–i12. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.A.; Gunukula, S.K.; Aleem, S.; Obeid, R.; Jaoude, P.A.; Honeine, R.; Irani, J. The prevalence of waterpipe tobacco smoking among the general and specific populations: A systematic review. BMC Public Health 2011, 11, 244. [Google Scholar] [CrossRef] [Green Version]
- Azab, M.; Khabour, O.F.; Alzoubi, K.; Anabtawi, M.M.; Quttina, M.; Khader, Y.; Eissenberg, T. Exposure of pregnant women to waterpipe and cigarette smoke. Nicotine Tob. Res. 2012, 15, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, S.; Mansournia, M.A.; Foroushani, A.R.; Mahmoodi, M.; Alavi, A.; Shekari, M.; Holakouie-Naieni, K. The effects of water-pipe smoking on birth weight: A population-based prospective cohort study in southern Iran. Epidemiol. Health 2018, 40, e2018008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maziak, W.; Ward, K.D.; Soweid, R.A.A.; Eissenberg, T. Tobacco smoking using a waterpipe: A re-emerging strain in a global epidemic. Tob. Control 2004, 13, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirahmadizadeh, A.; Nakhaee, N. Prevalence of waterpipe smoking among rural pregnant women in Southern Iran. Med. Princ. Pract. 2008, 17, 435–439. [Google Scholar] [CrossRef]
- Ashour, A.A.; Haik, M.Y.; Sadek, K.W.; Yalcin, H.C.; Bitharas, M.J.; Aboulkassim, T.; Batist, G.; Yasmeen, A.; Moustafa, A.-E.A. Substantial toxic effect of water-pipe smoking on the early stage of embryonic development. Nicotine Tob. Res. 2018, 20, 502–507. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, R.T.; MacRae, C.A. Systematic approaches to toxicology in the zebrafish. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.Z.; Benslimane, F.; Nasrallah, G.; Shurbaji, S.; Younes, N.N.; Mraiche, F.; Da’As, S.I.; Yalcin, H.C. Using Zebrafish for Investigating the Molecular Mechanisms of Drug-Induced Cardiotoxicity. BioMed Res. Int. 2018, 2018, 1642684. [Google Scholar] [CrossRef]
- Yalcin, H.C. Hemodynamic studies for analyzing the teratogenic effects of drugs in the zebrafish embryo. In Teratogenicity Testing; Springer: Berlin/Heidelberg, Germany, 2018; pp. 487–495. [Google Scholar]
- Chico, T.J.; Ingham, P.W.; Crossman, D.C. Modeling cardiovascular disease in the zebrafish. Trends Cardiovasc. Med. 2008, 18, 150–155. [Google Scholar] [CrossRef]
- Best, J.; Alderton, W.K. Zebrafish: An in vivo model for the study of neurological diseases. Neuropsychiatr. Dis. Treat. 2008, 4, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, H.M.; Zon, L.I. Cancer genetics and drug discovery in the zebrafish. Nat. Rev. Cancer 2003, 3, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.D.; Soo, E.C.; Achenbach, J.C.; Morash, M.G.; Soanes, K.H. Use of the zebrafish larvae as a model to study cigarette smoke condensate toxicity. PLoS ONE 2014, 9, e115305. [Google Scholar] [CrossRef]
- Aghamolaei, T.; Eftekhar, H.; Zare, S. Risk factors associated with intrauterine growth retardation (IUGR) in Bandar Abbas. J. Med. Sci. 2007, 7, 665–669. [Google Scholar] [CrossRef] [Green Version]
- Palpant, N.J.; Hofsteen, P.; Pabon, L.; Reinecke, H.; Murry, C.E. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PLoS ONE 2015, 10, e0126259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammah, M.; Dandachi, F.; Salman, R.; Shihadeh, A.; El-Sabban, M. In Vitro effects of waterpipe smoke condensate on endothelial cell function: A potential risk factor for vascular disease. Toxicol. Lett. 2013, 219, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Shihadeh, A.; Saleh, R. Polycyclic aromatic hydrocarbons, carbon monoxide,“tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem. Toxicol. 2005, 43, 655–661. [Google Scholar] [CrossRef]
- Sadek, K.W.; Haik, M.Y.; Ashour, A.A.; Baloch, T.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Zeidan, A.; Moustafa, A.-E.A. Water-pipe smoking promotes epithelial–mesenchymal transition and invasion of human breast cancer cells via ERK1/ERK2 pathways. Cancer Cell Int. 2018, 18, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.T.; Pomerantsev, E.V.; Mably, J.D.; MacRae, C.A. High-resolution cardiovascular function confirms functional orthology of myocardial contractility pathways in zebrafish. Physiol. Genom. 2010, 42, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, H.C.; Amindari, A.; Butcher, J.; Althani, A.; Yacoub, M. Heart Function and Hemodynamics Analysis for Zebrafish Embryos. Dev. Dyn. 2017, 246, 868–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benslimane, F.M.; Zakaria, Z.Z.; Shurbaji, S.; Abdelrasool, M.K.A.; Al-Badr, M.A.H.; Absi, E.S.K.A.; Yalcin, H.C. Cardiac function and blood flow hemodynamics assessment of zebrafish (Danio rerio) using high-speed video microscopy. Micron 2020, 136, 102876. [Google Scholar] [CrossRef]
- Benslimane, F.M.; Yalcin, H.C. Adaptation of a Mice Doppler Echocardiography Platform to measure cardiac flow velocities for embryonic chicken and adult Zebrafish. Front. Bioeng. Biotechnol. 2019, 7, 96. [Google Scholar] [CrossRef]
- Becker, J.R.; Chatterjee, S.; Robinson, T.Y.; Bennett, J.S.; Panáková, D.; Galindo, C.L.; Zhong, L.; Shin, J.T.; Coy, S.M.; Kelly, A.E.; et al. Differential activation of natriuretic peptide receptors modulates cardiomyocyte proliferation during development. Development 2014, 141, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.R.; Robinson, T.Y.; Sachidanandan, C.; Kelly, A.E.; Coy, S.; Peterson, R.T.; MacRae, C.A. In vivo natriuretic peptide reporter assay identifies chemical modifiers of hypertrophic cardiomyopathy signalling. Cardiovasc. Res. 2012, 93, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Narumanchi, S.; Wang, H.; Perttunen, S.; Tikkanen, I.; Lakkisto, P.; Paavola, J. Zebrafish Heart Failure Models. Front. Cell Dev. Biol. 2021, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.J.; Legleye, S.; Thomas, D.; Berlin, I. Tobacco smoking: The likely confounder of the association between heart diseases and suicide. J. Intern. Med. 2020, 288, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Podzolkov, V.I.; Bragina, A.E.; Druzhinina, N.A.; Vasil’Eva, L.V.; Osadchiy, K.K.; Dubchak, A.E.; Khvalin, E.I. Relation between Tobacco Smoking/Electronic Smoking and Albuminuria/Vascular Stiffness in Young People without Cardiovascular Diseases. Kidney Blood Press. Res. 2020, 45, 467–476. [Google Scholar] [CrossRef]
- Theilig, F.; Wu, Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol.-Ren. Physiol. 2015, 308, F1047–F1055. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.H.; Kim, H.W. Novel Antihypertension Mechanism of β-Glucan by Corin and ANP-Mediated Natriuresis in Mice. Mycobiology 2020, 48, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Chopra, S.; Cherian, D.; Verghese, P.P. Physiology and clinical significance of natriuretic hormones. Indian J. Endocrinol. Metab. 2013, 17, 83. [Google Scholar] [PubMed]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.G. Natriuretic peptides: Markers or modulators of cardiac hypertrophy? Trends Endocrinol. Metab. 2003, 14, 411–416. [Google Scholar] [CrossRef]
- Wang, D.; Oparil, S.; Feng, J.A.; Li, P.; Perry, G.; Chen, L.B.; Dai, M.; John, S.W.; Chen, Y.-F. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide–null mouse. Hypertension 2003, 42, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Chen, Y.-F.; Feng, J.A.; Hayashi, T.; Oparil, S.; Perry, G.J. Volume overload results in exaggerated cardiac hypertrophy in the atrial natriuretic peptide knockout mouse. Cardiovasc. Res. 2004, 61, 771–779. [Google Scholar] [CrossRef]
- Albert, I. Zebrafish as a Model System for Safety Pharmacology, Using a Glucocorticoid and an Antimicrobial Peptide as Test Substances. Master’s Thesis, Universitetet I Tromsø, Tromsø, Norway, 2013. [Google Scholar]
- Yabu, T.; Yabu, T.; Kishi, S.; Okazaki, T.; Yamashita, M. Characterization of zebrafish caspase-3 and induction of apoptosis through ceramide generation in fish fathead minnow tailbud cells and zebrafish embryo. Biochem. J. 2001, 360, 39–47. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, Z.Z.; Aladwi, S.A.; Benslimane, F.; Al-Absi, E.S.; Al-Shafai, M.; Yalcin, H.C.; Khalil, A.; Moustafa, A.-E.A.; Al-Asmakh, M. Effect of Water-Pipe Smoking on the Normal Development of Zebrafish. Int. J. Environ. Res. Public Health 2021, 18, 11659. https://doi.org/10.3390/ijerph182111659
Zakaria ZZ, Aladwi SA, Benslimane F, Al-Absi ES, Al-Shafai M, Yalcin HC, Khalil A, Moustafa A-EA, Al-Asmakh M. Effect of Water-Pipe Smoking on the Normal Development of Zebrafish. International Journal of Environmental Research and Public Health. 2021; 18(21):11659. https://doi.org/10.3390/ijerph182111659
Chicago/Turabian StyleZakaria, Zain Zaki, Shaima Ahmad Aladwi, Fatiha Benslimane, Enas S. Al-Absi, Mashael Al-Shafai, Huseyin C. Yalcin, Ashraf Khalil, Ala-Eddin Al Moustafa, and Maha Al-Asmakh. 2021. "Effect of Water-Pipe Smoking on the Normal Development of Zebrafish" International Journal of Environmental Research and Public Health 18, no. 21: 11659. https://doi.org/10.3390/ijerph182111659
APA StyleZakaria, Z. Z., Aladwi, S. A., Benslimane, F., Al-Absi, E. S., Al-Shafai, M., Yalcin, H. C., Khalil, A., Moustafa, A. -E. A., & Al-Asmakh, M. (2021). Effect of Water-Pipe Smoking on the Normal Development of Zebrafish. International Journal of Environmental Research and Public Health, 18(21), 11659. https://doi.org/10.3390/ijerph182111659