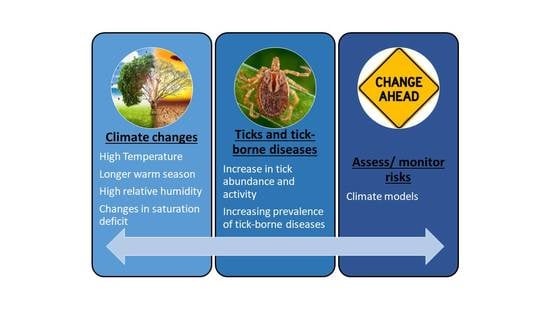
Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases
Abstract
:1. Introduction
2. How Climate Affects Tick Phenology—The Example of I. ricinus
| Country | Changes in I. ricinus Prevalence | Current Incidence of Tick-Borne Diseases | Future Incidence of Tick-Borne Diseases (by 2050) | Ref. | |
|---|---|---|---|---|---|
| Northern Europe | Czech Republic |
| Ιncreasing incidence of TBE | Ιncreasing incidence of TBE | [59] |
| Sweden |
| Ιncreasing incidence of LB | Ιncreasing incidence of tick-borne disease in southern parts of the country | [60] | |
| Norway |
| Decreasing incidence of TBE | Ιncreasing incidence of tick-borne disease in southern parts of the country | [60] | |
| Finland |
| Ιncreasing incidence of LB | Ιncreasing incidence of tick-borne disease in southern parts of the country | [60] | |
| Germany |
| Ιncreasing incidence of LB | Ιncreasing incidence of LB | [61] | |
| Southern Europe | Greece |
at higher latitude and/or altitudes | Low incidence of tick-borne diseases | Have reduced areas of high- risk incidence of tick-borne diseases | [60] |
| Italy |
at higher latitude and/or altitudes | Low incidence of tick-borne diseases | High-risk incidence of tick-borne diseases | [60] | |
| Portugal |
at higher latitude and/or altitudes | Low incidence of tick-borne diseases | Have reduced areas of high- risk incidence of tick-borne diseases | [60] |
3. The Effect of Climate Change on the Prevalence of LB and TBE
4. Climate Models as a Useful Tool in Predicting the Risk of Tick-Borne Diseases
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Černý, J.; Lynn, G.; Hrnková, J.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. Management Options for Ixodes ricinus-Associated Pathogens: A Review of Prevention Strategies. Int. J. Environ. Res. Public Health 2020, 17, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzoli, A.; Hauffe, H.C.; Carpi, G.; Vourc’h, G.I.; Neteler, M.; Rosà, R. Lyme borreliosis in Europe. Eurosurveillance 2011, 16, 19906. [Google Scholar] [CrossRef]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antiviral Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum-a widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grankvist, A.; Andersson, P.O.; Mattsson, M.; Sender, M.; Vaht, K.; Höper, L.; Sakiniene, E.; Trysberg, E.; Stenson, M.; Fehr, J.; et al. Infections with the tick-borne bacterium “Candidatus Neoehrlichia mikurensis” mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin. Infect. Dis. 2014, 58, 1716–1722. [Google Scholar] [CrossRef]
- Lejal, E.; Marsot, M.; Chalvet-Monfray, K.; Cosson, J.F.; Moutailler, S.; Vayssier-Taussat, M.; Pollet, T. A three-years assessment of Ixodes ricinus-borne pathogens in a French peri-urban forest. Parasites Vectors 2019, 12, 551. [Google Scholar] [CrossRef] [Green Version]
- Regier, Y.; Ballhorn, W.; Kempf, V.A.J. Molecular detection of Bartonella henselae in 11 Ixodes ricinus ticks extracted from a single cat. Parasites Vectors 2017, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Körner, S.; Makert, G.R.; Ulbert, S.; Pfeffer, M.; Mertens-Scholz, K. The Prevalence of Coxiella burnetii in Hard Ticks in Europe and Their Role in Q Fever Transmission Revisited—A Systematic Review. Front. Vet. Sci. 2021, 8, 655715. [Google Scholar] [CrossRef]
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013, 100, 159–189. [Google Scholar] [CrossRef] [Green Version]
- Patz, J.A.; Campbell-Lendrum, D.; Holloway, T.; Foley, J.A. Impact of regional climate change on human health. Nature 2005, 438, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Bittner, M.I.; Matthies, E.F.; Dalbokova, D.; Menne, B. Are European countries prepared for the next big heat-wave? Eur. J. Public Health 2014, 24, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porretta, D.; Mastrantonio, V.; Amendolia, S.; Gaiarsa, S.; Epis, S.; Genchi, C.; Bandi, C.; Otranto, D.; Urbanelli, S. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasites Vectors 2013, 6, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Sprong, H.; Hofhuis, A.; Gassner, F.; Takken, W.; Jacobs, F.; Van Vliet, A.J.H.; Van Ballegooijen, M.; Van Der Giessen, J.; Takumi, K. Circumstantial evidence for an increase in the total number and activity of borrelia-infected ixodes ricinus in the Netherlands. Parasites Vectors 2012, 5, 294. [Google Scholar] [CrossRef] [Green Version]
- Omazic, A.; Bylund, H.; Boqvist, S.; Högberg, A.; Björkman, C.; Tryland, M.; Evengård, B.; Koch, A.; Berggren, C.; Malogolovkin, A.; et al. Identifying climate-sensitive infectious diseases in animals and humans in Northern regions. Acta Vet. Scand. 2019, 61, 53. [Google Scholar] [CrossRef] [Green Version]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef]
- Randolph, S.E. Ticks and tick-borne disease systems in space and from space. Adv. Parasitol. 2000, 47, 217–243. [Google Scholar] [CrossRef]
- Capelli, G.; Ravagnan, S.; Montarsi, F.; Ciocchetta, S.; Cazzin, S.; Porcellato, E.; Babiker, A.M.; Cassini, R.; Salviato, A.; Cattoli, G.; et al. Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: A cost-effectiveness analysis in north-eastern Italy. Parasites Vectors 2012, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Mannelli, A.; Bertolotti, L.; Gern, L.; Gray, J. Ecology of Borrelia burgdorferi sensu lato in Europe: Transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol. Rev. 2012, 36, 837–861. [Google Scholar] [CrossRef] [Green Version]
- Randolph, S.E.; Green, R.M.; Peacey, M.F.; Rogers, D.J. Seasonal synchrony: The key to tick-borne encephalitis foci identified by satellite data. Parasitology 2000, 121, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Cull, B.; Pietzsch, M.E.; Hansford, K.M.; Gillingham, E.L.; Medlock, J.M. Surveillance of British ticks: An overview of species records, host associations, and new records of Ixodes ricinus distribution. Ticks Tick-Borne Dis. 2018, 9, 605–614. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Jaenson, D.G.E.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafiso, A.; Olivieri, E.; Floriano, A.M.; Chiappa, G.; Serra, V.; Sassera, D.; Bazzocchi, C. Investigation of tick-borne pathogens in ixodes ricinus in a peri-urban park in lombardy (Italy) reveals the presence of emerging pathogens. Pathogens 2021, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Akl, T.; Bourgoin, G.; Souq, M.L.; Appolinaire, J.; Poirel, M.T.; Gibert, P.; Abi Rizk, G.; Garel, M.; Zenner, L. Detection of tick-borne pathogens in questing Ixodes ricinus in the French Pyrenees and first identification of Rickettsia monacensis in France. Parasite 2019, 26, 20. [Google Scholar] [CrossRef] [Green Version]
- Remesar, S.; Fernández, P.D.; Venzal, J.M.; Pérez-Creo, A.; Prieto, A.; Estrada-Peña, A.; López, C.M.; Panadero, R.; Fernández, G.; Díez-Baños, P.; et al. Tick species diversity and population dynamics of Ixodes ricinus in Galicia (north-western Spain). Ticks Tick-Borne Dis. 2019, 10, 132–137. [Google Scholar] [CrossRef]
- Ben, I.; Lozynskyi, I. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus and Dermacentor reticulatus and Coinfection with Borrelia burgdorferi and Tick-Borne Encephalitis Virus in Western Ukraine. Vector Borne Zoonotic Dis. 2019, 19, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Zając, Z.; Kulisz, J.; Bartosik, K.; Woźniak, A.; Dzierżak, M.; Khan, A. Environmental determinants of the occurrence and activity of Ixodes ricinus ticks and the prevalence of tick-borne diseases in eastern Poland. Sci. Rep. 2021, 11, 15472. [Google Scholar] [CrossRef]
- Kalmár, Z.; Dumitrache, M.; D’Amico, G.; Matei, I.A.; Ionică, A.M.; Gherman, C.M.; Lupșe, M.; Mihalca, A.D. Multiple Tick-Borne Pathogens in Ixodes ricinus Ticks Collected from Humans in Romania. Pathogens 2020, 9, 390. [Google Scholar] [CrossRef]
- Rousseau, R.; Vanwambeke, S.O.; Boland, C.; Mori, M. The Isolation of Culturable Bacteria in Ixodes ricinus Ticks of a Belgian Peri-Urban Forest Uncovers Opportunistic Bacteria Potentially Important for Public Health. Int. J. Environ. Res. Public Health 2021, 18, 12134. [Google Scholar] [CrossRef] [PubMed]
- Capligina, V.; Seleznova, M.; Akopjana, S.; Freimane, L.; Lazovska, M.; Krumins, R.; Kivrane, A.; Namina, A.; Aleinikova, D.; Kimsis, J.; et al. Large-scale countrywide screening for tick-borne pathogens in field-collected ticks in Latvia during 2017–2019. Parasites Vectors 2020, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, M.; Radzijevskaja, J.; Mickevičius, S.; Bratčikovienė, N.; Paulauskas, A. Prevalence of tick-borne encephalitis virus in questing Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania. Ticks Tick-Borne Dis. 2021, 12, 101594. [Google Scholar] [CrossRef] [PubMed]
- Vogelgesang, J.R.; Walter, M.; Kahl, O.; Rubel, F.; Brugger, K. Long-term monitoring of the seasonal density of questing ixodid ticks in Vienna (Austria): Setup and first results. Exp. Appl. Acarol. 2020, 81, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ixodes Ricinus—Current Known Distribution: March 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/ixodes-ricinus-current-known-distribution-march-2021 (accessed on 22 July 2021).
- Efstratiou, A.; Karanis, G.; Karanis, P. Tick-Borne Pathogens and Diseases in Greece. Microorganisms 2021, 9, 1732. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E.; Randolph, S.; Randolph, S.E. IJNM Mini-Review Evidence that climate change has caused “emergence” of tick-borne diseases in Europe? Introduction -the undeniable impact of climate on tick-borne diseases. Int. J. Med. Microbiol. 2004, 293, 5–15. [Google Scholar]
- Gilbert, L. The Impacts of Climate Change on Ticks and Tick-Borne Disease Risk. Annu. Rev. Entomol. 2021, 66, 273–288. [Google Scholar] [CrossRef]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of Climate Change on Ticks and Tick-Borne Diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef]
- Alonso-Carné, J.; García-Martín, A.; Estrada-Peña, A. Modelling the Phenological Relationships of Questing Immature Ixodes Ricinus (Ixodidae) Using Temperature and NDVI Data. Zoonoses Public Health 2016, 63, 40–52. [Google Scholar] [CrossRef]
- Cat, J.; Beugnet, F.; Hoch, T.; Jongejan, F.; Prangé, A.; Chalvet-Monfray, K. Influence of the spatial heterogeneity in tick abundance in the modeling of the seasonal activity of Ixodes ricinus nymphs in Western Europe. Exp. Appl. Acarol. 2017, 71, 115–130. [Google Scholar] [CrossRef]
- Hauser, G.; Rais, O.; Morán Cadenas, F.; Gonseth, Y.; Bouzelboudjen, M.; Gern, L. Influence of climatic factors on Ixodes ricinus nymph abundance and phenology over a long-term monthly observation in Switzerland (2000–2014). Parasites Vectors 2018, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z.; Halouzka, J.; Juricová, Z. Host-seeking activity of ixodid ticks in relation to weather variables. J. Vector Ecol. 2003, 28, 159–165. [Google Scholar] [PubMed]
- Daniel, M.; Malý, M.; Danielová, V.; Kəíž, B.; Nuttall, P. Abiotic predictors and annual seasonal dynamics of Ixodes ricinus, the major disease vector of Central Europe. Parasites Vectors 2015, 8, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef]
- Tomkins, J.L.; Aungier, J.; Hazel, W.; Gilbert, L. Towards an evolutionary understanding of questing behaviour in the tick Ixodes ricinus. PLoS ONE 2014, 9, e110028. [Google Scholar] [CrossRef] [Green Version]
- Perret, J.L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef]
- Alasmari, S.; Wall, R. Metabolic rate and resource depletion in the tick Ixodes ricinus in response to temperature. Exp. Appl. Acarol. 2021, 83, 81–93. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355. [Google Scholar] [CrossRef]
- Daniel, M. Influence of the microclimate on the vertical distribution of the tick Ixodes ricinus (L) in Central Europe. Acarologia 1993, 34, 105–113. [Google Scholar]
- Burtis, J.C.; Sullivan, P.; Levi, T.; Oggenfuss, K.; Fahey, T.J.; Ostfeld, R.S. The impact of temperature and precipitation on blacklegged tick activity and lyme disease incidence in endemic and emerging regions. Parasites Vectors 2016, 9, 606. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, E.; Tälleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.; Talleklint, L.; Lundqvist, L.; Olsen, B.; Chirico, J.; Mejlon, H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J. Med. Entomol. 1994, 31, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Tälleklint, L.; Jaenson, T.G.T. Increasing Geographical Distribution and Density of Ixodes ricinus (Acari: Ixodidae) in Central and Northern Sweden. J. Med. Entomol. 1998, 35, 521–526. [Google Scholar] [CrossRef]
- Dautel, H.; Knülle, W. Cold hardiness, supercooling ability and causes of low-temperature mortality in the soft tick, Argas reflexus, and the hard tick, Ixodes ricinus (Acari: Ixodoidea) from Central Europe. J. Insect Physiol. 1997, 43, 843–854. [Google Scholar] [CrossRef]
- Gilbert, L. Altitudinal patterns of tick and host abundance: A potential role for climate change in regulating tick-borne diseases? Oecologia 2010, 162, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Jouda, F.; Perret, J.L.; Gern, L. Ixodes ricinus density, and distribution and prevalence of Borrelia burgdorferi sensu lato infection along an altitudinal gradient. J. Med. Entomol. 2004, 41, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Needham, G.R.; Teel, P.D. Off-host physiological ecology of ixodid ticks. Annu. Rev. Entomol. 1991, 36, 659–681. [Google Scholar] [CrossRef]
- Comparison of the Epidemiological Patterns of Lyme Borreliosis and Tick-Borne Encephalitis in the Czech Republic in 2007–2016—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30602281/ (accessed on 14 May 2022).
- Li, S.; Gilbert, L.; Vanwambeke, S.O.; Yu, J.; Purse, B.V.; Harrison, P.A. lyme disease risks in europe under multiple uncertain drivers of change. Environ. Health Perspect. 2019, 127, 067010-1-13. [Google Scholar] [CrossRef]
- Enkelmann, J.; Böhmer, M.; Fingerle, V.; Siffczyk, C.; Werber, D.; Littmann, M.; Merbecks, S.S.; Helmeke, C.; Schroeder, S.; Hell, S.; et al. Incidence of notified Lyme borreliosis in Germany, 2013–2017. Sci. Rep. 2018, 8, 14976. [Google Scholar] [CrossRef]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Vrijmoeth, H.D.; Van De Schoor, F.; Hovius, J.W. Lyme borreliosis: Diagnosis and management. BMJ 2020, 369, m1041. [Google Scholar] [CrossRef] [PubMed]
- James, M.C.; Bowman, A.S.; Forbes, K.J.; Lewis, F.; McLeod, J.E.; Gilbert, L. Environmental determinants of Ixodes ricinus ticks and the incidence of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Parasitology 2013, 140, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrulionienė, A.; Radzišauskienė, D.; Ambrozaitis, A.; Čaplinskas, S.; Paulauskas, A.; Venalis, A. Epidemiology of LB in a highly endemic European zone. Medicina 2020, 56, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Peña, A.; Ortega, C.; Sánchez, N.; DeSimone, L.; Sudre, B.; Suk, J.E.; Semenza, J.C. Correlation of Borrelia burgdorferi Sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the Western Palearctic. Appl. Environ. Microbiol. 2011, 77, 3838–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrama-Rakotoarivony, L.; Sudre, B.; Bortel, W.V.; Warns-Petit, E.; Zeller, H. Annual Epidemiological Report Emerging and Vector-Borne Diseases; ECDC: Stockholm, Sweden, 2014. [Google Scholar]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Sykes, R.A.; Makiello, P. An estimate of Lyme borreliosis incidence inWestern Europe. J. Public Health 2017, 39, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Jore, S.; Viljugrein, H.; Hofshagen, M.; Brun-Hansen, H.; Kristoffersen, A.B.; Nygård, K.; Brun, E.; Ottesen, P.; Sævik, B.K.; Ytrehus, B. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasites Vectors 2011, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Gocko, X.; Lenormand, C.; Lemogne, C.; Bouiller, K.; Gehanno, J.F.; Rabaud, C.; Perrot, S.; Eldin, C.; de Broucker, T.; Roblot, F.; et al. Lyme borreliosis and other tick-borne diseases. Guidelines from the French scientific societies. Med. Mal. Infect. 2019, 49, 296–317. [Google Scholar] [CrossRef]
- Zanzani, S.A.; Rimoldi, S.G.; Manfredi, M.T.; Grande, R.; Gazzonis, A.L.; Merli, S.; Olivieri, E.; Giacomet, V.; Antinori, S.; Cislaghi, G.; et al. Lyme borreliosis incidence in Lombardy, Italy (2000–2015): Spatiotemporal analysis and environmental risk factors. Ticks Tick-Borne Dis. 2019, 10, 101257. [Google Scholar] [CrossRef]
- Cairns, V.; Wallenhorst, C.; Rietbrock, S.; Martinez, C. Incidence of lyme disease in the UK: A population-based cohort study. BMJ Open 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Sajanti, E.; Virtanen, M.; Helve, O.; Kuusi, M.; Lyytikäinen, O.; Hytönen, J.; Sane, J. Lyme borreliosis in Finland, 1995–2014. Emerg. Infect. Dis. 2017, 23, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigfússon, H.B.; Haroarson, H.S.; Lúovíksson, B.R.; Guolaugsson, Ó. LB in Iceland—Epidemiology from 2011 to 2015. Laeknabladid 2019, 105, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, M.E.; Pego-Reigosa, R.; Díez-Morrondo, C.; Castro-Gago, M.; Díaz, P.; Fernández, G.; Morrondo, P. Epidemiology of LB in a healthcare area in north-west Spain. Gac. Sanit. 2015, 29, 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation; World Health Organization: Geneva, Switzerland, 2012; pp. 1–42. Available online: https://apps.who.int/iris/handle/10665/70809 (accessed on 1 April 2022).
- Amicizia, D.; Domnich, A.; Panatto, D.; Lai, P.L.; Cristina, M.L.; Avio, U.; Gasparini, R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccines Immunother. 2013, 9, 1163. [Google Scholar] [CrossRef] [Green Version]
- Petri, E.; Gniel, D.; Zent, O. Tick-borne encephalitis (TBE) trends in epidemiology and current and future management. Travel Med. Infect. Dis. 2010, 8, 233–245. [Google Scholar] [CrossRef]
- Nah, K.; Magpantay, F.M.G.; Bede-Fazekas, Á.; Röst, G.; Trájer, A.J.; Wu, X.; Zhang, X.; Wu, J. Assessing systemic and non-systemic transmission risk of tick-borne encephalitis virus in Hungary. PLoS ONE 2019, 14, e0217206. [Google Scholar] [CrossRef]
- Nah, K.; Bede-Fazekas, Á.; Trájer, A.J.; Wu, J. The potential impact of climate change on the transmission risk of tick-borne encephalitis in Hungary. BMC Infect. Dis. 2020, 20, 34. [Google Scholar] [CrossRef] [Green Version]
- Zeman, P. Prolongation of tick-borne encephalitis cycles in warmer climatic conditions. Int. J. Environ. Res. Public Health 2019, 16, 4532. [Google Scholar] [CrossRef] [Green Version]
- Randolph, S.E.; Rogers, D.J. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proc. R. Soc. B Biol. Sci. 2000, 267, 1741–1744. [Google Scholar] [CrossRef] [Green Version]
- Sumilo, D.; Asokliene, L.; Bormane, A.; Vasilenko, V.; Golovljova, I.; Randolph, S.E. Climate Change Cannot Explain the Upsurge of Tick-Borne Encephalitis in the Baltics. PLoS ONE 2007, 2, e500. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, E.; Gustafson, R. Tick-borne encephalitis in Sweden and climate change. Lancet 2001, 358, 16–18. [Google Scholar] [CrossRef]
- Daniel, M.; Danielová, V.; Fialová, A.; Malý, M.; Kříž, B.; Nuttall, P.A. Increased relative risk of tick-borne encephalitis in warmer weather. Front. Cell. Infect. Microbiol. 2018, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kříž, B.; Kott, I.; Daniel, M.; Vráblík, T. Beneš Vliv klimatických změn na výskyt onemocnění klíšťovou encefalitidou v letech 1982-2011 v České republice. Epidemiol. Mikrobiol. Imunol. 2015, 64, 24–32. [Google Scholar] [PubMed]
- Brabec, M.; Daniel, M.; Malý, M.; Danielová, V.; Kříž, B.; Kott, I.; Beneš, Č. Analysis of meteorological effects on the incidence of tick-borne encephalitis in the Czech Republic over a thirty-year period. Virol. Res. Rev. 2017, 1, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Home—NPHO EOΔΥ. Available online: https://eody.gov.gr/en/ (accessed on 22 July 2021).
- Tick-Borne Encephalitis. Available online: https://eody.gov.gr/disease/krotonogenis-egkefalitida/ (accessed on 25 July 2021).
- Baylis, M. Potential impact of climate change on emerging vector-borne and other infections in the UK. Environ. Health Glob. Access Sci. Source 2017, 16, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Lukan, M.; Bullova, E.; Petko, B. Climate warming and tick-borne encephalitis, Slovakia. Emerg. Infect. Dis. 2010, 16, 524–526. [Google Scholar] [CrossRef]
- Mills, J.N.; Gage, K.L.; Khan, A.S. Potential influence of climate change on vector-borne and zoonotic diseases: A review and proposed research plan. Environ. Health Perspect. 2010, 118, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, E.; Andersson, Y.; Suk, J.E.; Sudre, B.; Semenza, J.C. Public health: Monitoring EU emerging infectious disease risk due to climate change. Science 2012, 336, 418–419. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, U.; Peterson, A.T.; Chaudhry, M.N.; Ashraf, I.; Saqib, Z.; Ahmad, S.R.; Ali, H.; Ashraf, C.; Peterson, A.T.; Chaudhry, M.N.; et al. Ecological niche model comparison under different climate scenarios: A case study of Olea spp. in Asia. Ecosphere 2017, 8, e01825. [Google Scholar] [CrossRef]
- Li, S.; Gilbert, L.; Harrison, P.A.; Rounsevell, M.D.A. Modelling the seasonality of lyme disease risk and the potential impacts of a warming climate within the heterogeneous landscapes of Scotland. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Boeckmann, M.; Joyner, T.A. Old health risks in new places? An ecological niche model for I. ricinus tick distribution in Europe under a changing climate. Health Place 2014, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.K.; Townsend Peterson, A.; Cobos, M.E.; Ganta, R.; Foley, D. Current and Future Distribution of the Lone Star Tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef] [PubMed]
- Alkishe, A.A.; Peterson, A.T.; Samy, A.M. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS ONE 2017, 12, e0189092. [Google Scholar] [CrossRef] [PubMed]
- Bregnard, C.; Rais, O.; Voordouw, M.J. Climate and tree seed production predict the abundance of the European LB vector over a 15-year period. Parasites Vectors 2020, 13, 408. [Google Scholar] [CrossRef] [PubMed]
- Nolzen Id, H.; Brugger, K.; Reichold, A.; Brock, J.; Lange, M.; Thulkeid, H.-H. Model-based extrapolation of ecological systems under future climate scenarios: The example of Ixodes ricinus ticks. PLoS ONE 2022, 17, e0267196. [Google Scholar] [CrossRef]
- Donša, D.; Grujić, V.J.; Pipenbaher, N.; Ivajnšič, D. The lyme borreliosis spatial footprint in the 21st century: A key study of slovenia. Int. J. Environ. Res. Public Health 2021, 18, 12061. [Google Scholar] [CrossRef]
- Uusitalo, R.; Siljander, M.; Dub, T.; Sane, J.; Sormunen, J.J.; Pellikka, P.; Vapalahti, O. Modelling habitat suitability for occurrence of human tick-borne encephalitis (TBE) cases in Finland. Ticks Tick-Borne Dis. 2020, 11, 101457. [Google Scholar] [CrossRef]
| Region | Incidence Rate (100,000 People per Year) | Reference |
|---|---|---|
| Western Europe | 22.05 cases | [69] |
| France (2009–2017) | 53 cases | [71] |
| Northern Italy (2000–2015) | 12.4 cases | [72] |
| United Kingdom | 12.1 cases | [73] |
| Finland | 61 cases | [74] |
| Iceland | 2 cases | [75] |
| Spain | 2.5–11.6 cases | [76] |
| Lithuania | 99.9 cases | [65] |
| Germany | 400 cases | [77] |
| Regions of Slovenia, Austria, Baltic Coastline of Northern Sweden, some Estonian and Finnish Islands | 100 cases | [2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voyiatzaki, C.; Papailia, S.I.; Venetikou, M.S.; Pouris, J.; Tsoumani, M.E.; Papageorgiou, E.G. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. Int. J. Environ. Res. Public Health 2022, 19, 6516. https://doi.org/10.3390/ijerph19116516
Voyiatzaki C, Papailia SI, Venetikou MS, Pouris J, Tsoumani ME, Papageorgiou EG. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. International Journal of Environmental Research and Public Health. 2022; 19(11):6516. https://doi.org/10.3390/ijerph19116516
Chicago/Turabian StyleVoyiatzaki, Chrysa, Sevastiani I. Papailia, Maria S. Venetikou, John Pouris, Maria E. Tsoumani, and Effie G. Papageorgiou. 2022. "Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases" International Journal of Environmental Research and Public Health 19, no. 11: 6516. https://doi.org/10.3390/ijerph19116516
APA StyleVoyiatzaki, C., Papailia, S. I., Venetikou, M. S., Pouris, J., Tsoumani, M. E., & Papageorgiou, E. G. (2022). Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. International Journal of Environmental Research and Public Health, 19(11), 6516. https://doi.org/10.3390/ijerph19116516