Energy Consumption in Capacitive Deionization for Desalination: A Review
Abstract
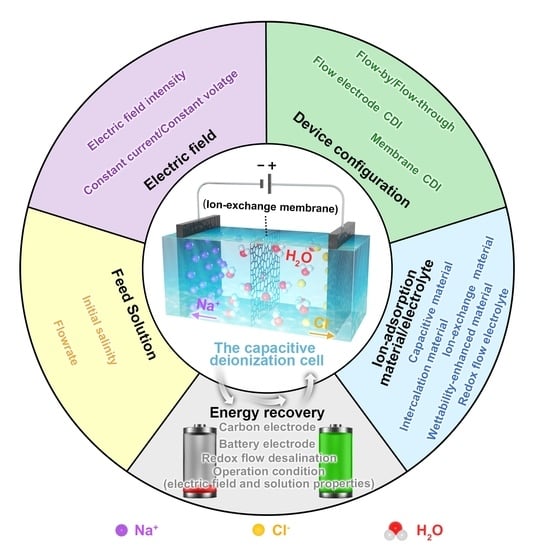
:1. Introduction
2. The Impacts of the Device Configuration on the Energy Consumption of CDI
2.1. Membrane CDI
2.2. Flow Electrode CDI
2.3. Flow-By and Flow-Through Cells
3. The Impacts of the Electrical Field and Feed Solution Properties on the Energy Consumption of CDI
3.1. Charging Modes
3.2. Electric Field Intensity
3.3. Flow Rate
3.4. Initial Salt Concentration
4. The Impacts of the Electrode Materials on the Energy Consumption of CDI
4.1. Capacitive Materials
4.2. Intercalation Materials
4.3. Ion-Exchange Materials
4.4. Materials with High Wettability
4.5. Ion-Adsorption Electrolytes in Redox Flow Desalination
5. The Energy Recovery of CDI Desalination
5.1. Energy Recovery of CDI with Carbon Electrodes
5.2. Energy Recovery of CDI with Battery Electrodes
5.3. Energy Recovery of Redox Flow Desalination
5.4. The Effect of Operation Conditions on the Energy Recovery of CDI
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lu, T.; Liu, Y.; Xu, X.T.; Pan, L.K.; Alothman, A.A.; Shapter, J.; Wang, Y.; Yamauchi, Y. Highly efficient water desalination by capacitive deionization on biomass-derived porous carbon nanoflakes. Sep. Purif. Technol. 2021, 256, 117771. [Google Scholar] [CrossRef]
- Xing, W.L.; Liang, J.; Tang, W.W.; He, D.; Yan, M.; Wang, X.X.; Luo, Y.; Tang, N.; Huang, M. Versatile applications of capacitive deionization (CDI)-based technologies. Desalination 2020, 482, 114390. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of Capacitive Deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar]
- Pan, S.Y.; Snyder, S.W.; Lin, Y.J.; Chiang, P.C. Electrokinetic desalination of brackish water and associated challenges in the water and energy nexus. Environ. Sci.-Wat. Res. Technol. 2018, 4, 613–638. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Landon, J.; Gao, X.; Omosebi, A.; Liu, K.L. Progress and outlook for capacitive deionization technology. Curr. Opin. Chem. Eng. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.M.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J. A protic salt-derived porous carbon for efficient capacitive deionization: Balance between porous structure and chemical composition. Carbon 2017, 116, 21–32. [Google Scholar] [CrossRef]
- Tian, S.C.; Zhang, Z.H.; Zhang, X.H.; Ostrikov, K. Capacitative deionization using commercial activated carbon fiber decorated with polyaniline. J. Colloid Interface Sci. 2019, 537, 247–255. [Google Scholar] [CrossRef]
- Ding, Z.B.; Xu, X.T.; Li, Y.Q.; Wang, K.; Lu, T.; Pan, L.K. Significantly improved stability of hybrid capacitive deionization using nickel hexacyanoferrate/reduced graphene oxide cathode at low voltage operation. Desalination 2019, 468, 114078. [Google Scholar] [CrossRef]
- Liu, X.T.; Shanbhag, S.; Mauter, M.S. Understanding and mitigating performance decline in electrochemical deionization. Curr. Opin. Chem. Eng. 2019, 25, 67–74. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Yue, Z.S.; Li, H.B. Na0.71CoO2 promoted sodium uptake via faradaic reaction for highly efficient capacitive deionization. Sep. Purif. Technol. 2020, 234, 116090. [Google Scholar] [CrossRef]
- Zhou, R.J.; Wang, M.Y.; Wei, W.H.; Li, J.X.; Tong, Y.X.; Liang, B.; Chen, F.M.; Liu, G.W.; Luo, M. Enhanced Desalination Capacity and Stability of Alkylamine- Modified Na-0.71 CoO2 for Capacitive Deionization. ACS Sustain. Chem. Eng. 2021, 9, 1949–1957. [Google Scholar] [CrossRef]
- Moustafa, H.M.; Obaid, M.; Nassar, M.M.; Abdelkareem, M.A.; Mahmoud, M.S. Titanium dioxide-decorated rGO as an effective electrode for ultrahigh-performance capacitive deionization. Sep. Purif. Technol. 2020, 235, 116178. [Google Scholar] [CrossRef]
- Yue, Z.S.; Gao, T.; Li, H.B. Robust synthesis of carbon@Na4Ti9O20 core-shell nanotubes for hybrid capacitive deionization with enhanced performance. Desalination 2019, 449, 69–77. [Google Scholar] [CrossRef]
- Huang, Y.X.; Chen, F.M.; Guo, L.; Yang, H.Y. Ultrahigh performance of a novel electrochemical deionization system based on a NaTi2(PO4)3/rGO nanocomposite. J. Mater. Chem. A 2017, 5, 18157–18165. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Y.H.; An, Z.H.; Xu, G.R.; Gadgil, A.J.; Ruan, G.L. The polymeric conformational effect on capacitive deionization performance of graphene oxide/polypyrrole composite electrode. Desalination 2020, 486, 114407. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.G.; Xu, S.C.; Liu, Q.; Wang, J.X. Polypyrrole/polyaniline composites with enhanced performance for capacitive deionization. Desalin. Water Treat. 2015, 54, 3248–3256. [Google Scholar] [CrossRef]
- Bao, W.Z.; Tang, X.; Guo, X.; Choi, S.; Wang, C.Y.; Gogotsi, Y.; Wang, G.X. Porous Cryo-Dried MXene for Efficient Capacitive Deionization. Joule 2018, 2, 778–787. [Google Scholar] [CrossRef]
- Guo, L.; Mo, R.W.; Shi, W.H.; Huang, Y.X.; Leong, Z.Y.; Ding, M.; Chen, F.M.; Yang, H.Y. A Prussian blue anode for high performance electrochemical deionization promoted by the faradaic mechanism. Nanoscale 2017, 9, 13305–13312. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Ding, Z.B.; Li, Y.Q.; Lu, T.; Pan, L.K. Metal-organic-frameworks-derived NaTi2(PO4)3/carbon composites for efficient hybrid capacitive deionization. J. Mater. Chem. A 2019, 7, 12126–12133. [Google Scholar] [CrossRef]
- Ma, J.; Xiong, Y.C.; Dai, X.H.; Yu, F. Zinc Spinel Ferrite Nanoparticles as a Pseudocapacitive Electrode with Ultrahigh Desalination Capacity and Long-Term Stability. Environ. Sci. Technol. Lett. 2020, 7, 118–125. [Google Scholar] [CrossRef]
- Chen, B.B.; Feng, A.H.; Deng, R.X.; Liu, K.; Yu, Y.; Song, L.X. MXene as a Cation-Selective Cathode Material for Asymmetric Capacitive Deionization. ACS Appl. Mater. Interfaces 2020, 12, 13750–13758. [Google Scholar] [CrossRef]
- Hackl, L.; Tsai, S.W.; Kalyan, B.; Hou, C.H.; Gadgil, A. Electrically regenerated ion-exchange technology: Leveraging faradaic reactions and assessing the effect of co-ion sorption. J. Colloid Interface Sci. 2022, 623, 985–991. [Google Scholar] [CrossRef]
- Liu, P.Y.; Wang, H.; Yan, T.T.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Grafting sulfonic and amine functional groups on 3D graphene for improved capacitive deionization. J. Mater. Chem. A 2016, 4, 5303–5313. [Google Scholar] [CrossRef]
- Wu, T.T.; Wang, G.; Dong, Q.; Qian, B.Q.; Meng, Y.L.; Qiu, J.S. Asymmetric capacitive deionization utilizing nitric acid treated activated carbon fiber as the cathode. Electrochim. Acta 2015, 176, 426–433. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, K.K.; Eum, H.M.; Lee, C.W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, J.H. Enhanced desalination efficiency in capacitive deionization with an ion-selective membrane. Sep. Purif. Technol. 2010, 71, 70–75. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, Y.; Wang, R.G.; Wu, Y.F.; Xu, S.C.; Wang, J.X. Performance comparison and energy consumption analysis of capacitive deionization and membrane capacitive deionization processes. Desalination 2013, 324, 127–133. [Google Scholar] [CrossRef]
- Zhang, C.Y.; He, D.; Ma, J.X.; Tang, W.W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)–problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Lee, H.; Yoon, H.; Shin, D.; Ahn, W.; Cho, N.; Han, U.; Hong, J.; Tran, N.A.T.; Yoo, C.Y.; et al. Enhanced salt removal performance of flow electrode capacitive deionization with high cell operational potential. Sep. Purifoy. Technol. 2021, 254, 117500. [Google Scholar] [CrossRef]
- Jeon, S.I.; Park, H.R.; Yeo, J.G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Ma, J.X.; Ma, J.J.; Zhang, C.Y.; Song, J.K.; Dong, W.J.; Waite, T.D. Flow-electrode capacitive deionization (FCDI) scale-up using a membrane stack configuration. Water Res. 2020, 168, 115186. [Google Scholar] [CrossRef]
- Hand, S.; Shang, X.; Guest, J.S.; Smith, K.C.; Cusick, R.D. Global Sensitivity Analysis To Characterize Operational Limits and Prioritize Performance Goals of Capacitive Deionization Technologies. Environ. Sci. Technol. 2019, 53, 3748–3756. [Google Scholar] [CrossRef]
- Ma, J.X.; He, C.; He, D.; Zhang, C.Y.; Waite, T.D. Analysis of capacitive and electrodialytic contributions to water desalination by flow-electrode CDI. Water Res. 2018, 144, 296–303. [Google Scholar] [CrossRef]
- Remillard, E.M.; Shocron, A.N.; Rahill, J.; Suss, M.E.; Vecitis, C.D. A direct comparison of flow-by and flow-through capacitive deionization. Desalination 2018, 444, 169–177. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Comparison of faradaic reactions in flow-through and flow-by capacitive deionization (CDI) systems. Electrochim. Acta 2019, 299, 727–735. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Hong, S. Staged voltage mode in membrane capacitive deionization: Comparison with constant voltage and constant current modes. Desalination 2020, 479, 114327. [Google Scholar] [CrossRef]
- Kang, J.; Kim, T.; Jo, K.; Yoon, J. Comparison of salt adsorption capacity and energy consumption between constant current and constant voltage operation in capacitive deionization. Desalination 2014, 352, 52–57. [Google Scholar] [CrossRef]
- Choi, J.-H. Comparison of constant voltage (CV) and constant current (CC) operation in the membrane capacitive deionisation process. Desalin. Water Treat. 2015, 56, 921–928. [Google Scholar] [CrossRef]
- Wang, L.; Lin, S.H. Membrane Capacitive Deionization with Constant Current vs. Constant Voltage Charging: Which Is Better? Environ. Sci. Technol. 2018, 52, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Chen, F.M.; Guo, L.; Zhang, J.; Chen, T.P.; Yang, H.Y. Low energy consumption dual-ion electrochemical deionization system using NaTi2(PO4)3-AgNPs electrodes. Desalination 2019, 451, 241–247. [Google Scholar] [CrossRef]
- Wang, L.; Dykstra, J.E.; Lin, S. Energy Efficiency of Capacitive Deionization. Environ. Sci. Technol. 2019, 53, 3366–3378. [Google Scholar] [CrossRef]
- Wang, L.; Lin, S.H. Intrinsic tradeoff between kinetic and energetic efficiencies in membrane capacitive deionization. Water Res. 2018, 129, 394–401. [Google Scholar] [CrossRef]
- Chen, K.Y.; Shen, Y.Y.; Wang, D.M.; Hou, C.H. Carbon nanotubes/activated carbon hybrid as a high-performance suspension electrode for the electrochemical desalination of wastewater. Desalination 2022, 522, 115440. [Google Scholar] [CrossRef]
- Halabaso, E.R.; Salvacion, J.W.L.; Kuncoro, E.P.; Doong, R.A. Highly efficient capacitive deionization of brackish water with manganese vanadate nanorod decorated reduced graphene oxide electrode. Environ.-Sci. Nano 2021, 8, 2844–2854. [Google Scholar] [CrossRef]
- Vafakhah, S.; Saeedikhani, M.; Tanhaei, M.; Huang, S.; Guo, L.; Chiam, S.Y.; Yang, H.Y. An energy efficient bi-functional electrode for continuous cation-selective capacitive deionization. Nanoscale 2020, 12, 22917–22927. [Google Scholar] [CrossRef]
- Xing, S.Y.; Cheng, Y.J.; Yu, F.; Ma, J. y Na3(VO)2(PO4)2F nanocuboids/graphene hybrid materials as faradic electrode for extra-high desalination capacity. J. Colloid Interface Sci. 2021, 598, 511–518. [Google Scholar] [CrossRef]
- Reale, E.R.; Shrivastava, A.; Smith, K.C. Effect of conductive additives on the transport properties of porous flow-through electrodes with insulative particles and their optimization for Faradaic deionization. Water Res. 2019, 165, 114995. [Google Scholar] [CrossRef]
- Pan, S.Y.; Haddad, A.Z.; Kumar, A.; Wang, S.W. Brackish water desalination using reverse osmosis and capacitive deionization at the water-energy nexus. Water Res. 2020, 183, 116064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Porada, S.; Biesheuvel, P.M.; Van der Wal, A. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. [Google Scholar] [CrossRef]
- Qin, M.H.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Patel, S.K.; Qin, M.; Walker, W.S.; Ehmelech, M. Energy Efficiency of Electro-Driven Brackish Water Desalination: Electrodialysis Significantly Outperforms Membrane Capacitive Deionization. Environ. Sci. Technol. 2020, 54, 3663–3677. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; van der Wal, A. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 2012, 5, 9520–9527. [Google Scholar] [CrossRef]
- Nakayama, Y.; Imamura, E.; Noda, S. Capacitive deionization characteristics of compressed granular activated carbon. Sep. Purif. Technol. 2021, 277, 119454. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Huang, Z.H.; Cui, T.X.; Gui, X.C.; Kang, F.Y.; Wang, K.L.; Wu, D.H. Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes. J. Mater. Chem. 2011, 21, 18295–18299. [Google Scholar] [CrossRef]
- Yang, S.; Park, H.R.; Yoo, J.; Kim, H.; Choi, J.; Han, M.H.; Kim, D.K. Plate-Shaped Graphite for Improved Performance of Flow-Electrode Capacitive Deionization. J. Electrochem. Soc. 2017, 164, E480–E488. [Google Scholar] [CrossRef]
- Al Radi, M.; Sayed, E.T.; Alawadhi, H.; Abdelkareem, M.A. Progress in energy recovery and graphene usage in capacitive deionization. Crit. Rev. Environ. Sci. Technol. 2021, 52, 3080–3136. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Zhang, X.M.; Liu, R.M.; Yang, Q.; Hu, S.; Song, H.O.; Li, P.C.; Li, A.M.; Zhang, S.P. Enhanced capacitive deionization of a low-concentration brackish water with protonated carbon nitride-decorated graphene oxide electrode. Chemosphere 2022, 293, 133580. [Google Scholar] [CrossRef]
- Kalfa, A.; Penki, T.R.; Cohen, I.; Shpigel, N.; Avraham, E.; Aurbach, D.; Liang, D.W.; Wu, Q.H.; Wang, H.N.; Xiang, Y. Thermally reduced graphene oxide as an electrode for CDI processes: A compromise between performance and scalability? Desalination 2020, 492, 114599. [Google Scholar] [CrossRef]
- Jung, H.H.; Hwang, S.W.; Hyun, S.H.; Kang-Ho, L.; Kim, G.T. Capacitive deionization characteristics of nanostructured carbon aerogel electrodes synthesized via ambient drying. Desalination 2007, 216, 377–385. [Google Scholar] [CrossRef]
- Han, L.; Karthikeyan, K.G.; Gregory, K.B. Energy Consumption and Recovery in Capacitive Deionization Using Nanoporous Activated Carbon Electrodes. J. Electrochem. Soc. 2015, 162, E282–E288. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Kang, J.; Min, J.; Kim, S.I.; Kim, S.W.; Jang, J.H. Three-level micro-meso-macroporous three-dimensional graphene for highly fast capacitive deionization. Mater. Today Energy 2020, 18, 100502. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Heil, D.; Wang, G. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res. 2008, 42, 2605–2617. [Google Scholar] [CrossRef]
- Sriramulu, D.; Yang, H.Y. Free-standing flexible film as a binder-free electrode for an efficient hybrid deionization system. Nanoscale 2019, 11, 5896–5908. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.; Yoon, J. Hybrid capacitive deionization with Ag coated carbon composite electrode. Desalination 2017, 422, 42–48. [Google Scholar] [CrossRef]
- Yin, H.J.; Zhao, S.L.; Wan, J.W.; Tang, H.J.; Chang, L.; He, L.C.; Zhao, H.J.; Gao, Y.; Tang, Z.Y. Three-Dimensional Graphene/Metal Oxide Nanoparticle Hybrids for High-Performance Capacitive Deionization of Saline Water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
- Lee, J.; Srimuk, P.; Zwingelstein, R.; Zornitta, R.L.; Choi, J.; Kim, C.; Presser, V. Sodium ion removal by hydrated vanadyl phosphate for electrochemical water desalination. J. Mater. Chem. A 2019, 7, 4175–4184. [Google Scholar] [CrossRef]
- Vafakhah, S.; Guo, L.; Sriramulu, D.; Huang, S.; Saeedikhani, M.; Yang, H.Y. Efficient sodium-ion intercalation into the freestanding Prussian blue/graphene aerogel anode in a hybrid capacitive deionization system. ACS Appl. Mater. Interfaces 2019, 11, 5989–5998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, X.; Wang, Z.; Wang, K.; Dou, X.; Zhu, H.; Yuan, X.; Pan, L. Controlled synthesis of bismuth oxychloride-carbon nanofiber hybrid materials as highly efficient electrodes for rocking-chair capacitive deionization. Chem. Eng. J. 2021, 403, 126326. [Google Scholar] [CrossRef]
- Srimuk, P.; Husmann, S.; Presser, V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater. RSC Adv. 2019, 9, 14849–14858. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.L.; Hu, Y.S.; Ji, X.L.; Chen, W. NASICON-Structured Materials for Energy Storage. Adv. Mater. 2017, 29, 1601925. [Google Scholar] [CrossRef]
- Wu, M.G.; Ni, W.; Hu, J.; Ma, J.M. NASICON-Structured NaTi2(PO4)3 for Sustainable Energy Storage. Nano-Micro Lett. 2019, 11, 44. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Zhang, Z.N.; Tang, Y.G.; Jia, C.K.; Ji, X.B.; Wang, H.Y. Understanding crystal structures, ion diffusion mechanisms and sodium storage behaviors of NASICON material. Energy Storage Mater. 2021, 34, 171–193. [Google Scholar] [CrossRef]
- Cao, J.L.; Wang, Y.; Wang, L.; Yu, F.; Ma, J. Na3V2(PO4)3@C as Faradaic Electrodes in Capacitive Deionization for High-Performance Desalination. Nano Lett. 2019, 19, 823–828. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Dong, X.; Hou, M.; Wang, Y.; Xia, Y. Integrating Desalination and Energy Storage via Saltwater-based Hybrid Sodium-ion Supercapacitor. Chemsuschem 2018, 11, 1741–1745. [Google Scholar] [CrossRef]
- Liu, B.; Yu, L.; Yu, F.; Ma, J. In-situ formation of uniform V2O5 nanocuboid from V2C MXene as electrodes for capacitive deionization with higher structural stability and ion diffusion ability. Desalination 2021, 500, 114897. [Google Scholar] [CrossRef]
- Santos, C.; Rodriguez, I.V.; Lado, J.J.; Vila, M.; Garcia-Quismondo, E.; Anderson, M.A.; Palma, J.; Vilatela, J.J. Low-energy consumption, free-form capacitive deionization through nanostructured networks. Carbon 2021, 176, 390–399. [Google Scholar] [CrossRef]
- Guo, L.; Kong, D.Z.; Pam, M.E.; Huang, S.Z.; Ding, M.; Shang, Y.; Gu, C.D.; Huang, Y.X.; Yang, H.Y. The efficient faradaic Li4Ti5O12@C electrode exceeds the membrane capacitive desalination performance. J. Mater. Chem. A 2019, 7, 8912–8921. [Google Scholar] [CrossRef]
- Dixit, F.; Zimmermann, K.; Dutta, R.; Prakash, N.J.; Barbeau, B.; Mohseni, M.; Kandasubramanian, B. Application of MXenes for water treatment and energy-efficient desalination: A review. J. Hazard. Mater. 2022, 423, 127050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Agren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Ma, J.; Cheng, Y.; Wang, L.; Dai, X.; Yu, F. Free-standing Ti3C2Tx MXene film as binder-free electrode in capacitive deionization with an ultrahigh desalination capacity. Chem. Eng. J. 2020, 384, 123329. [Google Scholar] [CrossRef]
- Kim, T.; Gorski, C.A.; Logan, B.E. Low energy desalination using battery electrode deionization. Environ. Sci. Technol. Lett. 2017, 4, 444–449. [Google Scholar] [CrossRef]
- Porada, S.; Shrivastava, A.; Bukowska, P.; Biesheuvel, P.M.; Smith, K.C. Nickel Hexacyanoferrate Electrodes for Continuous Cation Intercalation Desalination of Brackish Water. Electrochim. Acta 2017, 255, 369–378. [Google Scholar] [CrossRef]
- Srimuk, P.; Wang, L.; Budak, O.; Presser, V. High-performance ion removal via zinc-air desalination. Electrochem. Commun. 2020, 115, 106713. [Google Scholar] [CrossRef]
- Srimuk, P.; Lee, J.; Tolosa, A.; Kim, C.; Aslan, M.; Presser, V. Titanium disulfide: A promising low-dimensional electrode material for sodium ion intercalation for sea water desalination. Chem. Mater. 2017, 29, 9964–9973. [Google Scholar] [CrossRef]
- Liang, M.; Wang, L.; Presser, V.; Dai, X.; Yu, F.; Ma, J. Combining Battery-Type and Pseudocapacitive Charge Storage in Ag/Ti3C2Tx MXene Electrode for Capturing Chloride Ions with High Capacitance and Fast Ion Transport. Adv. Sci. 2020, 7, 2000621. [Google Scholar] [CrossRef]
- Ma, J.; Wang, L.; Yu, F.; Dai, X.H. Mesoporous amorphous FePO4 nanosphere@Graphene as a faradic electrode in capacitive deionization for high-capacity and fast removal of NaCl from water. Chem. Eng. J. 2019, 370, 938–943. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Liu, Y.; Khan, I.U.; Gong, A.; Huo, S.L.; Li, K.X. Synergistic Improvement in Capacitive Deionization Performance Using a Novel Phase-Integrated Na0.55Mn2O4@Na0.7MnO2. ACS Sustain. Chem. Eng. 2021, 9, 2496–2506. [Google Scholar] [CrossRef]
- Arnold, S.; Wang, L.; Budak, Z.; Aslan, M.; Presser, V. Antimony alloying electrode for high-performance sodium removal: How to use a battery material not stable in aqueous media for saline water remediation. J. Mater. Chem. A 2020, 9, 585–596. [Google Scholar] [CrossRef]
- ul Haq, O.; Choi, J.H.; Lee, Y.S. Synthesis of ion-exchange polyaniline-carbon composite electrodes for capacitive deionization. Desalination 2020, 479, 114308. [Google Scholar] [CrossRef]
- Wu, Q.H.; Liang, D.W.; Avraham, E.; Cohen, I.; Aurbach, D.; Lu, S.F.; Wang, H.N.; Xiang, Y. Enhanced capacitive deionization of an integrated membrane electrode by thin layer spray-coating of ion exchange polymers on activated carbon electrode. Desalination 2020, 491, 114460. [Google Scholar] [CrossRef]
- Belaustegui, Y.; Zorita, S.; Fernandez-Carretero, F.; Garcia-Luis, A.; Panto, F.; Stelitano, S.; Frontera, P.; Antonucci, P.; Santangelo, S. Electro-spun graphene-enriched carbon fibres with high nitrogen-contents for electrochemical water desalination. Desalination 2018, 428, 40–49. [Google Scholar] [CrossRef]
- Yan, T.; Liu, J.; Lei, H.; Shi, L.; An, Z.; Park, H.S.; Zhang, D. Capacitive deionization of saline water using sandwich-like nitrogen-doped graphene composites via a self-assembling strategy. Environ.-Sci. Nano 2018, 5, 2722–2730. [Google Scholar] [CrossRef]
- Wei, Z.; Xwa, B.; Xza, B.; Sha, B.; Ao, G.; Xma, B.; Kla, B. Well-dispersed Prussian blue analogues connected with carbon nanotubes for efficient capacitive deionization process. Sep. Purif. Technol. 2022, 287, 120483. [Google Scholar]
- Hou, X.H.; Liang, Q.; Hu, X.Q.; Zhou, Y.; Ru, Q.; Chen, F.M.; Hu, S.J. Coupling desalination and energy storage with redox flow electrodes. Nanoscale 2018, 10, 12308–12314. [Google Scholar] [CrossRef]
- Srimuk, P.; Su, X.; Yoon, J.; Aurbach, D.; Presser, V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 2020, 5, 517–538. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Feng, C.; Ma, J.; Waite, T.D. Low energy consumption and mechanism study of redox flow desalination. Chem. Eng. J. 2020, 401, 126111. [Google Scholar] [CrossRef]
- Ramalingam, K.; Zhu, Y.C.; Wang, J.; Liang, M.J.; Wei, Q.; Chen, X.C.; Sun, F.; Chen, D.Y.; Zhang, Z.; Aung, S.H.; et al. Efficient PEDOT Electrode Architecture for Continuous Redox-Flow Desalination. ACS Sustain. Chem. Eng. 2021, 9, 12779–12787. [Google Scholar] [CrossRef]
- Kang, J.; Kim, T.; Shin, H.; Lee, J.; Ha, J.I.; Yoon, J. Direct energy recovery system for membrane capacitive deionization. Desalination 2016, 398, 144–150. [Google Scholar] [CrossRef]
- Shiue, L.; Hung, W.; Chou, G.; Wang, S.; Chung, H.; Chiu, I. Energy-Effective Desalination with Online Sterilization Using Capacitors. In Proceedings of the IDA World Congress, Singapore, 13 September 2005. [Google Scholar]
- Pernia, A.M.; Norniella, J.G.; Martin-Ramos, J.A.; Diaz, J.; Martinez, J.A. Up-Down Converter for Energy Recovery in a CDI Desalination System. IEEE Trans. Power Electron. 2012, 27, 3257–3265. [Google Scholar] [CrossRef]
- Andres, G.L.; Yoshihara, Y. A capacitive deionization system with high energy recovery and effective re-use. Energy 2016, 103, 605–617. [Google Scholar] [CrossRef]
- Dlugolecki, P.; Wal, A. Energy Recovery in Membrane Capacitive Deionization. Environ. Sci. Technol. 2013, 47, 4904–4910. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Linnartz, C.J.; Mueller, D.; Willenberg, L.K.; Wessling, M. Energy Recovery and Process Design in Continuous Flow Electrode Capacitive Deionization Processes. ACS Sustain. Chem. Eng. 2018, 6, 13007–13015. [Google Scholar] [CrossRef]
- Tan, C.; He, C.; Tang, W.W.; Kovalsky, P.; Fletcher, J.; Waite, T.D. Integration of photovoltaic energy supply with membrane capacitive deionization (MCDI) for salt removal from brackish waters. Water Res. 2018, 147, 276–286. [Google Scholar] [CrossRef]
- Tan, C.; He, C.; Fletcher, J.; Waite, T.D. Energy recovery in pilot scale membrane CDI treatment of brackish waters. Water Res. 2020, 168, 115146. [Google Scholar] [CrossRef]
- Chen, F.; Huang, Y.; Kong, D.; Ding, M.; Huang, S.; Yang, H.Y. NaTi2(PO4)3-Ag electrodes based desalination battery and energy recovery. FlatChem 2018, 8, 9–16. [Google Scholar] [CrossRef]
- Guo, L.; Shang, Y.; Wang, G.; Jin, J.; Leong, Z.Y.; Huang, S.; Gu, C.; Ding, M.; Pam, M.E.; Vafakhah, S. A membrane-less desalination battery with ultrahigh energy efficiency. J. Mater. Chem. A 2021, 9, 7216–7226. [Google Scholar] [CrossRef]
- Wang, Z.D.; Tian, S.H.; Niu, J.J.; Kong, W.; Lin, J.Y.; Hao, X.G.; Guan, G.Q. An electrochemically switched ion exchange process with self-electrical-energy recuperation for desalination. Sep. Purif. Technol. 2020, 239, 116521. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Desai, D.; Beh, E.S.; Sahu, S.; Vedharathinam, V.; van Overmeere, Q.; de Lannoy, C.F.; Jose, A.P.; Volkel, A.R.; Rivest, J.B. Electrochemical Desalination of Seawater and Hypersaline Brines with Coupled Electricity Storage. ACS Energy Lett. 2018, 3, 375–379. [Google Scholar] [CrossRef]
- Khalla, S.A.; Suss, M.E. Desalination via chemical energy: An electrodialysis cell driven by spontaneous electrode reactions. Desalination 2019, 467, 257–262. [Google Scholar] [CrossRef]
- Qian, L.; Chen, F.; Wang, S.; Qiang, R.; Shi, Y. An organic flow desalination battery. Energy Storage Mater. 2018, 20, 203–207. [Google Scholar]
- Kim, B.; Seo, J.Y.; Chung, C. A hybrid system of capacitive deionization and redox flow battery for continuous desalination and energy storage. J. Power Sources 2020, 448, 227384. [Google Scholar] [CrossRef]
- Debruler, C.; Wu, W.; Cox, K.; Vanness, B.; Liu, T.L. Integrated Saltwater Desalination and Energy Storage through a pH Neutral Aqueous Organic Redox Flow Battery. Adv. Funct. Mater. 2020, 30, 2000385. [Google Scholar] [CrossRef]
- Son, M.; Pothanamkandathil, V.; Yang, W.L.; Vrouwenvelder, J.S.; Gorski, C.A.; Logan, B.E. Improving the Thermodynamic Energy Efficiency of Battery Electrode Deionization Using Flow-Through Electrodes. Environ. Sci. Technol. 2020, 54, 3628–3635. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chen, J.F.; Lin, C.H.; Hou, C.H. Integrating a supercapacitor with capacitive deionization for direct energy recovery from the desalination of brackish water. Appl. Energy 2019, 252, 1113417. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Hong, S.W.; Kim, C.; Yoon, J. Parametric study of multichannel desalination battery for low-energy electrochemical deionization of brackish water. Desalination 2021, 515, 115188. [Google Scholar] [CrossRef]
- Nam, D.H.; Lumley, M.A.; Choi, K.S. A Desalination Battery Combining Cu3[Fe(CN)6]2 as a Na-Storage Electrode and Bi as a Cl-Storage Electrode Enabling Membrane-Free Desalination. Chem. Mat. 2019, 31, 1460–1468. [Google Scholar] [CrossRef]
- Zornitta, R.L.; Ruotolo, L.A.M. Simultaneous analysis of electrosorption capacity and kinetics for CDI desalination using different electrode configurations. Chem. Eng. J. 2018, 332, 33–41. [Google Scholar] [CrossRef]
- Kim, T.; Dykstra, J.E.; Porada, S.; van der Wal, A.; Yoon, J.; Biesheuvel, P.M. Enhanced charge efficiency and reduced energy use in capacitive deionization by increasing the discharge voltage. J. Colloid Interface Sci. 2015, 446, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, J.E.; Porada, S.; van der Wal, A.; Biesheuvel, P.M. Energy consumption in capacitive deionization—Constant current versus constant voltage operation. Water Res. 2018, 143, 367–375. [Google Scholar] [CrossRef]
- Sriramulu, D.; Vafakhah, S.; Yang, H.Y. Activated Luffa derived biowaste carbon for enhanced desalination performance in brackish water. Rsc. Adv. 2019, 9, 14884–14892. [Google Scholar] [CrossRef] [Green Version]






| Material Couple | Charging Mode | Voltage/Current Density and Voltage Window | Energy Consumption | Initial Salinity | Desalination Capacity | Charge Efficiency | Reference |
|---|---|---|---|---|---|---|---|
| Ag || NaTi2(PO4)3/rGO | CC | 100 mA·g−1 (0~1.4 V) | about 0.18 Wh·gNaCl−1 | 2500 ppm | 35.8 mg·g−1 | --- | [43] |
| Na3(VO)2(PO4)2/rGO || AC | CC | 25 mA·g−1 (−1.4~1.4 V) | 0.35 Wh·gNaCl−1 | 1000 ppm | 175.94 mg·g−1 | --- | [49] |
| NiCHF || NiCHF | CC | 1 mA·cm−2 | 0.033 Wh·gNaCl−1 | 100 mM | 54.5–60 mg·g−1 | about 86% | [50] |
| Fe4[Fe(CN)6]3/rGO || rGO | CC | 100 mA·g−1 (−0.2~1.4 V) | 0.23 Wh·gNaCl−1 | 2500 ppm | 80 mg·g−1 | --- | [72] |
| BiOCl/C || Bi/C | CC | --- | 0.23 Wh·gNaCl−1 | 20 mM | --- | --- | [73] |
| AgCl || Ag | CC | 100 mA·g−1 (−0.1~0.1 V) | 0.059 Wh·gNaCl−1 | 600 mM | 115 mg·g−1 | 98% | [74] |
| Na3V2(PO4)3/C || AC | CV | 1.0 V | 0.46 Wh·gNaCl−1 | 100 mM | 137.20 mg·g−1 | 98.7% | [78] |
| AC || NaTi2(PO4)3/C | CC | 10 mA·cm−2 (0~2.0 V) | 0.11 Wh·gNaCl−1 | 600 mM | --- | --- | [79] |
| Na2VTi(PO4)3 || Na2VTi(PO4)3 | CC | 75 mA·g−1 (−0.1~0.1 V) | 0.068 Wh·gNaCl−1 | 1000 ppm | 90 mg·g−1 | --- | [48] |
| V2O5 || AC | CC | 30 mA·g−1 (−0.4~0.8 V) | 0.16 Wh·gNaCl−1 | 500 ppm | 22.3 mg·g−1 | --- | [80] |
| γAl2O3/CNT || TiO2/CNT | CC | 7.5 mA·g−1 (0~1.2 V) | 0.18 Wh·gNaCl−1 | 10 mM | 12.7 mg·g−1 | 85% | [81] |
| Carbon cloth || Li4Ti5O12/C | CC | 0.16 mA·cm−2 (−1.4~1.4 V) | 0.57 Wh·gNaCl−1 | 2500 ppm | 25 mg·g−1 | 83% | [82] |
| MXene Ti3C2Tx || MXene Ti3C2Tx | CC | 20 mA·g−1 (−1.2~1.2 V) | 0.24 Wh·gNaCl−1 | 585 ppm | 68 mg·g−1 | --- | [85] |
| CuCHF || CuCHF | CC | 1.4 A·m−2 (−0.6~0.6 V) | 0.01 Wh·gNaCl−1 | 25 mM | --- | --- | [86] |
| Na2NiFe(CN)6|| NaNiFe(CN)6 | CC | 1.4 A·m−2 (−1.5~1.5V) | 0.26 Wh·gNaCl−1 | 20 mM | about 27 mg·g−1 | 95% | [87] |
| MoS2 || Zn | CC | 1.4 mA·cm−2 (0~3 V) | 1.57 Wh·gNaCl−1 | 600 mM | 1300 mg·g−1 | 70% | [88] |
| TiS2 || Carbon textile | CC | 100 mA·g−1 (0~1.2 V) | 0.68 Wh·gNaCl−1 | 600 mM | 14.5 mg·g−1 | >85% | [89] |
| Ti3C2Tx || Ti3C2Tx/Ag | CC | 50 mA·g−1 (about −1.2–1.2 V) | 0.26 Wh·gNaCl−1 | 10 mM | 128.40 mg·g−1 | --- | [90] |
| FePO4/rGO || rGO | CV | 1.8 V | 0.9 Wh·gNaCl−1 | 40 mM | 85.94 mg·g−1 | 91.4% | [91] |
| Na0.55Mn2O4/Na0.7MnO2 || Na0.55Mn2O4/Na0.7MnO2 | CV | 1.0 V | 0.55 Wh·gNaCl−1 | 50 mM | 68.5 mg·g−1 | 84% | [92] |
| Sb || Porous carbon | CC | 200 mA·g−1 (−2.0~2.0 V) | 0.67 Wh·gNaCl−1 | 600 mM | 748 mg·g−1 | 74% | [93] |
| Material/Electrolyte Couple | Initial Salinity | Energy Recovery Mode | Regeneration Electric Intensity | Energy Recovery Rate | Energy Consumption with Energy Recovery | Reference |
|---|---|---|---|---|---|---|
| Carbon || Carbon | --- | frequency control | --- | 84% | --- | [105] |
| Activated charcoal || Activated charcoal | 5.5 mS·cm−1 | short-circuiting | --- | 70% | --- | [106] |
| Porous carbon || Porous carbon | 273 mM | CC | 1.69 A·m−2 | about 84% | 0.44 Wh·gNaCl−1 | [107] |
| AC || AC (flow electrode) | 60,000 ppm | CC | 2.48 mA·cm−2 | 36.2% | 0.44 Wh·gNaCl−1 | [108] |
| Carbon || Carbon | 4000 ppm | CC | --- | 62% | --- | [109] |
| Carbon || Carbon | 1900 ppm | CC | --- | about 40% | 0.57 Wh·gNaCl−1 | [110] |
| AC || AC | 50 mM | short-circuiting | --- | 49.6% | --- | [121] |
| Fe4[Fe(CN)6]3/rGO || rGO | 2500 ppm | CC | 100 mA·g−1 | 39% | about 0.15 Wh·gNaCl−1 | [72] |
| Ag/rGO || NaTi2(PO4)3/rGO | 2500 ppm | CC | 100 mA·g−1 | over 30% | 0.13 Wh·gNaCl−1 | [43] |
| Ag/CNT || NaTi2(PO4)3/graphene | 35,000 ppm | CC | 1000 mA·g−1 | 71.9% | 0.11 Wh·gNaCl−1 | [111] |
| MXene Ti3C2Tx || MXene Ti3C2Tx | 10 mM | CC | 20 mA·g−1 | 5.44% | 0.23 Wh·gNaCl−1 | [85] |
| Ni, Co MOF/black phorsphorus || Ag/rGO | synthetic seawater | CC | 300 mA·g−1 | 70.7% | 0.034 Wh·gNaCl−1 | [112] |
| Iron hexacyanoferrate || Polypyrrole/SO42− | 30,339 ppm | CC | 1.88 mA·cm−2 | 65% | 0.0089 Wh·gNaCl−1 | [113] |
| CuCHF || CuCHF | 50 mM | CC | 5 A·m−2 | 51% | 0.017 Wh·gNaCl−1 | [120] |
| NiCHF || Ag | 50 mM | CC | 5 A·m−2 | 73% | --- | [122] |
| CuCHF || Bi | 0.6 M | CC | 1 mA·cm−2 | 75.6% | --- | [123] |
| NaI/NaI3 || VCl2/VCl3 (redox flow) | about 19,000 ppm | CC | 0.22 mA·cm−2 | 52.4% | 0.092 Wh·gNaCl−1 | [99] |
| K4Fe(CN)6/K3Fe(CN)6 || ZnCl2 (redox flow) | 35,000 ppm | CC | 2.48 mA·cm−2 | over 80% | 0.070 Wh·gNaCl−1 | [115] |
| Br2/NaBr || ZnCl2 (redox flow) | 29,220 ppm | CC | 2 mA·cm−2 | 85% | 0.13 Wh·gNaCl−1 | [116] |
| TEMPO || FMN-Na (redox flow) | 1 M | CC | 0.13 mA·cm−2 | 25% | --- | [117] |
| PTIO || PTIO (redox flow) | 50 mM | CC | 5 mA·cm−2 | --- | 1.04 Wh·gNaCl−1 | [118] |
| Na4Fe(CN)6 || methyl viologen (redox flow) | 560 mM | CC | 1.33 mA·cm−2 | 79.7% | 0.070 Wh·gNaCl−1 | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Jin, L.; Wei, D.; Alhassan, S.I.; Wang, H.; Chai, L. Energy Consumption in Capacitive Deionization for Desalination: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10599. https://doi.org/10.3390/ijerph191710599
Jiang Y, Jin L, Wei D, Alhassan SI, Wang H, Chai L. Energy Consumption in Capacitive Deionization for Desalination: A Review. International Journal of Environmental Research and Public Health. 2022; 19(17):10599. https://doi.org/10.3390/ijerph191710599
Chicago/Turabian StyleJiang, Yuxin, Linfeng Jin, Dun Wei, Sikpaam Issaka Alhassan, Haiying Wang, and Liyuan Chai. 2022. "Energy Consumption in Capacitive Deionization for Desalination: A Review" International Journal of Environmental Research and Public Health 19, no. 17: 10599. https://doi.org/10.3390/ijerph191710599
APA StyleJiang, Y., Jin, L., Wei, D., Alhassan, S. I., Wang, H., & Chai, L. (2022). Energy Consumption in Capacitive Deionization for Desalination: A Review. International Journal of Environmental Research and Public Health, 19(17), 10599. https://doi.org/10.3390/ijerph191710599