The Effect of Short Treatment with Nigella Sativa on Symptoms, the Cluster of Differentiation (CD) Profile, and Inflammatory Markers in Mild COVID-19 Patients: A Randomized, Double-Blind Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
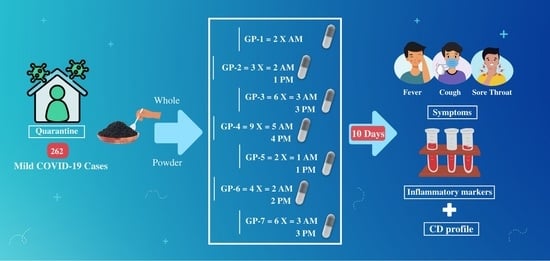
2.2. Nigella sativa Preparation
2.3. Determination of Sample Size
2.4. Study Population
2.5. Randomization and Blindness
2.6. The Primary Outcome
2.7. The Secondary Outcome
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef] [PubMed]
- Maideen, N.M.P. Prophetic Medicine-Nigella Sativa (Black cumin seeds)—Potential herb for COVID-19? J. Pharmacopunct. 2020, 23, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Chu, D.-T.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother. Res. 35, 1329–1344. [CrossRef]
- Kulyar, M.F.-A.; Li, R.; Mehmood, K.; Waqas, M.; Li, K.; Li, J. Potential influence of Nigella sativa (Black cumin) in reinforcing immune system: A hope to decelerate the COVID-19 pandemic. Phytomedicine 2021, 85, 153277. [Google Scholar] [CrossRef]
- Hannan, A.; Rahman, A.; Sohag, A.A.M.; Uddin, J.; Dash, R.; Sikder, M.H.; Rahman, S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Salem, M.L.; Hossain, M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int. J. Immunopharmacol. 2000, 22, 729–740. [Google Scholar] [CrossRef]
- Ulasli, M.; Gurses, S.A.; Bayraktar, R.; Yumrutas, O.; Oztuzcu, S.; Igci, M.; Igci, Y.Z.; Cakmak, E.A.; Arslan, A. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extract on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014, 41, 1703–1711. [Google Scholar] [CrossRef]
- Sajid, U.; Saqib, A.S.; Shah, M.A.; Khan, M.I.; Munir, M.T.; Nisa, Q.; Subhan, S.; Azam, T.; Umar, W.; Rehman, Z. Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys. J. Saudi Soc. Agric. Sci. 2016. [Google Scholar] [CrossRef]
- Oyero, O.G.; Toyama, M.; Mitsuhiro, N.; Onifade, A.A.; Hidaka, A.; Okamoto, M.; Baba, M. Selective inhibition of hepatitis C virus replication by alpha-zam, a Nigella sativa seed formulation. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 144–148. [Google Scholar] [CrossRef]
- Barakat, E.M.F.; Wakeel, L.M.E.; Hagag, R.S. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J. Gastroenterol. 2013, 19, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Onifade, A.A.; Jewell, A.P.; Adedeji, W.A. Nigella Sativa Concoction Induced Sustained Seroreversion in HIV Patient. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 332–335. [Google Scholar] [CrossRef]
- Hadi, V.; Kheirouri, S.; Alizadeh, M.; Khabbazi, A.; Hosseini, H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Avicenna J. Phytomed. 2016, 6, 34–43. [Google Scholar] [PubMed]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: A randomized double-blind, placebo-controlled clinical trial. J. Clin. Lipidol. 2016, 10, 1203–1211. [Google Scholar] [CrossRef]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics; Brooks/Cole: Pacific Grove, CA, USA; Cengage Learning: Boston, MA, USA, 2011. [Google Scholar]
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on Clinical Trials of Black Seed (Nigella sativa) and Its Active Constituent, Thymoquinone. J. Pharmacopunct. 2017, 20, 179–193. [Google Scholar] [CrossRef]
- Salem, E.M.; Yar, T.; Bamosa, A.O.; Al-Quorain, A.; Yasawy, M.I.; Alsulaiman, R.M.; Randhawa, M.A. Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J. Gastroenterol. 2010, 16, 207–214. [Google Scholar] [CrossRef]
- Ministry of Health. COVID-19 Coronavirus Disease Guidelines. 2020. Available online: https://covid19.cdc.gov.sa/wp-content/uploads/2020/10/EN_COVID_19_Coronavirus_Disease_Guidelines_v2.0.pdf (accessed on 15 April 2020).
- Khazdair, M.R.; Ghafari, S.; Sadeghi, M. Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19. Pharm. Biol. 2021, 59, 696–701. [Google Scholar] [CrossRef]
- Fatima Shad, K.; Soubra, W.; Cordato, D.J. The role of thymoquinone, a major constituent of Nigella sativa, in the treatment of inflammatory and infectious diseases. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1445–1453. [Google Scholar] [CrossRef]
- Mahboubi, M. Natural therapeutic approach of Nigella sativa (Black seed) fixed oil in management of Sinusitis. Integr. Med. Res. 2018, 7, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Ashraf, S.; Ashraf, M.; Imran, M.A.; Kalsoom, L.; Siddiqui, U.N.; Farooq, I.; Habib, Z.; Ashraf, S.; Ghufran, M.; et al. Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multi-center placebo-controlled randomized clinical trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Bamosa, A.O.; Kaatabi, H.; Lebdaa, F.M.; Elq, A.-M.A.; Al-Sultanb, A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharmacol. 2010, 54, 344–354. [Google Scholar] [PubMed]
- Ardakani Movaghati, M.R.; Yousefi, M.; Saghebi, S.A.; Sadeghi Vazin, M.; Iraji, A.; Mosavat, S.H. Efficacy of black seed (Nigella sativa L.) on kidney stone dissolution: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2019, 33, 1404–1412. [Google Scholar] [CrossRef]
- Akhondian, J.; Parsa, A.; Rakhshande, H. The effect of Nigella sativa L. (black cumin seed) on intractable pediatric seizures. Med. Sci. Monit. 2007, 13, CR555-9. [Google Scholar]
- Shoaei-Hagh, P.; Kamelan Kafi, F.; Najafi, S.; Zamanzadeh, M.; Heidari Bakavoli, A.; Ramezani, J.; Soltanian, S.; Asili, J.; Hosseinzadeh, H.; Eslami, S.; et al. A randomized, double-blind, placebo-controlled, clinical trial to evaluate the benefits of Nigella sativa seeds oil in reducing cardiovascular risks in hypertensive patients. Phytother. Res. 2021, 35, 4388–4400. [Google Scholar] [CrossRef]
- Onifade, A.A.; Jewel, A.P.; Okesina, A.B. Virologic and Immunologic Outcome of Treatment of HIV Infection with a Herbal Concoction, A-ZAM, Among Clients Seeking Herbal Remedy in Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 37–44. [Google Scholar] [CrossRef]
- Onifade, A.A.; Jewell, A.P.; Ajadi, T.A.; Rahamon, S.K.; Ogunrin, O.O. Effectiveness of a herbal remedy in six HIV patients in Nigeria. J. Herb. Med. 2013, 3, 99–103. [Google Scholar] [CrossRef]
- Bin Abdulrahman, K.A.; Bamosa, A.O.; Aseri, K.S.; Bukhari, A.I.; Masuadi, E.M. Clinical Presentation of Asymptomatic and Mild SARS-CoV-2 Infection in Riyadh, Saudi Arabia. J. Multidiscip. Healthc. 2021, 14, 1341–1347. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: Indications of progression of disease. Ann. Hematol. 2020, 99, 1421–1428. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Zhong, J.; Zhou, Y.; Tang, Z.; Zhou, H.; He, J.; Mei, X.; Tang, Y.; Lin, B.; et al. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nat. Commun. 2021, 12, 1724. [Google Scholar] [CrossRef]
- Quast, I.; Tarlinton, D. B cell memory: Understanding COVID-19. Immunity 2021, 54, 205–210. [Google Scholar] [CrossRef]


| Demographic Characteristics | Total (n) | Age (Years) | Gender | Nationality | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Male | Female | Saudi | Non-Saudi | ||
| Groups | 262 | 35.2 ± 11.4 | 183 | 79 | 172 | 90 |
| Group-1 (Control) | 25 | 36.1 ± 9.6 | 18 (72.0) | 7 (28.0) | 12 (48.0) | 13 (52.0) |
| Group-2 (NS-W1) | 36 | 36.0 ± 9.5 | 28 (77.8) | 8 (22.2) | 20 (55.6) | 16 (44.4) |
| Group-3 (NS-W2) | 38 | 33.1 ± 11.3 | 29 (76.3) | 9 (23.7) | 27 (71.1) | 11 (28.9) |
| Group-4 (NS-W3) | 45 | 36.0 ± 11.8 | 29 (64.4) | 16 (35.6) | 28 (62.2) | 17 (37.8) |
| Group-5 (NS-G1) | 32 | 32.6 ± 14.4 | 20 (62.5) | 12 (37.5) | 26 (81.2) | 6 (18.8) |
| Group-6 (NS-G2) | 44 | 38.1 ± 12.4 | 30 (68.2) | 14 (31.8) | 29 (65.9) | 15 (34.1) |
| Group-7 (NS-G3) | 42 | 33.9 ± 13.2 | 29 (69.0) | 13 (31.0) | 30 (71.4) | 12 (28.6) |
| Sig. | p = 0.345 | p = 0.757 | p = 0.130 | |||
| Symptoms | Days | Group | ||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | ||
| Fever | 1 | 4 (16.0) | 10 (27.8) | 2 (5.3) | 7 (15.6) | 3 (9.4) | 5 (11.4) | 6 (14.3) |
| 5 | 3 (15.8) | 2 (6.0) | 1 (2.9) | 3 (7.0) | 1 (3.7) | 1 (2.4) | 1 (3.0) | |
| 10 | 0 (0.0) | 0 (0.0) b | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cough | 1 | 7 (28.0) | 14 (38.9) | 14 (36.8) | 13 (28.9) | 10 (31.2) | 13 (29.5) | 14 (33.3) |
| 5 | 6 (31.6) | 7 (21.2) | 10 (28.6) | 12 (27.9) | 8 (29.6) | 15 (36.6) | 9 (27.3) | |
| 10 | 3 (17.6) | 3 (13.0) | 5 (16.7) | 7 (18.4) | 4 (16.0) | 9 (24.3) | 7 (26.9) | |
| Sense of smell | 1 | 8 (32.0) | 14 (38.9) | 9 (23.7) | 18 (40.0) | 17 (53.1) | 20 (45.5) | 17 (40.5) |
| 5 | 4 (21.1) | 13 (39.4) | 16 (45.7) | 16 (37.2) | 11 (40.7) | 15 (36.6) | 15 (45.5) | |
| 10 | 1 (5.9) | 4 (17.4) | 5 (16.7) | 8 (21.1) b | 7 (28.0) b | 8 (21.6) b | 8 (30.8) | |
| Lose sense of Taste | 1 | 6 (24.0) | 11 (30.6) | 9 (23.7) | 15 (33.3) | 17 (53.1) | 20 (45.5) | 15 (35.7) |
| 5 | 4 (21.1) | 11 (33.3) | 15 (42.9) | 14 (32.6) | 11 (40.7) | 11 (26.8) | 10 (30.3) | |
| 10 | 1 (5.9) b | 3 (13.0) | 4 (13.3) | 7 (18.4) | 4 (16.0) b | 7 (18.9) b | 3 (14.8) | |
| Shortening of breath | 1 | 3 (12.0) | 2 (5.6) | 3 (7.9) | 5 (11.1) | 3 (9.4) | 4 (9.1) | 4 (9.5) |
| 5 | 2 (10.5) | 3 (9.1) | 0 (0.0) | 3 (7.0) | 2 (7.4) | 2 (4.9) | 3 (9.1) | |
| 10 | 1 (5.9) | 0 (0.0) | 0 (0.0) | 1 (2.6) | 1 (4.0) | 2 (5.4) | 0 (0.0) | |
| Nasal Discharge | 1 | 4 (16.0) | 9 (25.0) | 6 (15.8) | 10 (22.2) | 3 (9.4) | 9 (20.5) | 6 (14.3) |
| 5 | 6 (31.6) | 4 (12.1) | 8 (22.9) | 5 (11.6) | 4 (14.8) | 6 (14.6) | 4 (12.1) | |
| 10 | 0 (0.0) b | 2 (8.7) | 3 (10.0) | 4 (10.5) | 2 (8.0) | 1 (2.7) | 1 (3.8) | |
| Sore Throat | 1 | 1 (4.0) | 8 (22.2) | 7 (18.4) | 10 (22.2) | 5 (15.6) | 5 (11.4) | 3 (7.1) |
| 5 | 1 (5.3) | 2 (6.1) | 2 (5.7) | 9 (20.9) | 5 (18.5) | 6 (14.6) | 1 (3.0) | |
| 10 | 1 (5.9) | 0 (0.0) b | 0 (0.0) | 3 (7.9) | 3 (12.0) | 1 (2.7) | 1 (3.8) | |
| General fatigue | 1 | 15 (16.0) | 16 (44.4) | 16 (42.1) | 21 (46.7) | 8 (25.0) | 20 (45.5) | 18 (42.9) |
| 5 | 6 (31.6) | 6 (18.2) | 7 (20.0) | 12 (27.9) | 5 (18.5) | 14 (34.1) | 8 (24.2) | |
| 10 | 1 (5.9) | 2 (8.7) b | 0 (0.0) b | 3 (7.9) b | 1 (4.0) b | 2 (5.4) b | 3 (11.5) b | |
| Headache | 1 | 7 (28.0) | 18 (50.0) | 17 (44.7) | 20 (44.4) | 11 (34.4) | 16 (36.4) | 8 (19.0) |
| 5 | 4 (21.1) | 6 (18.2) | 7 (20.0) | 11 (25.6) | 5 (18.5) | 10 (24.4) | 7 (21.2) | |
| 10 | 1 (5.9) b | 1 (4.3) | 2 (6.7) b | 3 (7.9) b | 4 (16.0) | 1 (2.7) b | 4 (15.4) | |
| Inflammatory Markers | Before vs. after Treatment Tests (Mean ± S.D) | Post-Test (ANOVA) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group-1 (n = 19) | Group-2 (n = 22) | Group-3 (n = 26) | Group-4 (n = 40) | Group-5 (n = 20) | Group-6 (n = 34) | Group-7 (n = 26) | |||
| ProCalcitonin (ng/mL) | Before | 0.06 ± 0.04 | 0.05 ± 0.04 | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.02 ± 0.00 | 0.04 ± 0.03 | 0.06 ± 0.07 | F = 1.11 p = 0.382 * |
| After | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 | ||
| Sig. | 0.014 ** | 0.357 | 0.106 | 0.005 ** | 0.829 | 0.004 ** | 0.228 | ||
| Ferritin (µg/L) | Before | 139.4 ± 93.5 | 127.8 ± 134.4 | 164.5 ± 170.2 | 104.1 ± 119.6 | 81.2 ± 82.6 | 124.7 ± 105.8 | 122.7 ± 153.1 | F = 0.866 p = 0.587 * |
| After | 135.8 ± 105.8 | 153.3 ± 165.6 | 135.3 ± 147.5 | 106.4 ± 102.4 | 94.1 ± 119.6 | 150.9 ± 158.1 | 101.9 ± 112.1 | ||
| Sig. | 0.097 | 0.381 | 0.664 | 0.971 | 0.176 | 0.150 | 0.632 | ||
| CRP (mg/L) | Before | 7.02 ± 8.13 | 10.7 ± 14.6 | 12.1 ± 13.51 | 7.66 ± 6.67 | 11.1 ± 16.0 | 7.04 ± 5.37 | 6.90 ± 7.69 | F = 2.1 p = 0.903 * |
| After | 7.55 ± 5.44 | 7.45 ± 3.37 | 6.09 ± 2.83 | 8.52 ± 7.27 | 6.53 ± 7.31 | 7.10 ± 4.79 | 7.13 ± 7.75 | ||
| Sig. | 0.893 | 0.191 | 0.071 | 0.705 | 0.442 | 0.570 | 0.790 | ||
| ESR (mm/h) | Before | 15.9 ± 11.97 | 14.3 ± 11.8 | 17.6 ± 17.6 | 18.45 ± 17.8 | 14.5 ± 13.4 | 16.3 ± 12.9 | 23.1 ± 25.1 | F = 0.55 p = 0.755 * |
| After | 16.2 ± 18.7 | 16.3 ± 16.8 | 17.4 ± 19.0 | 20.2 ± 21.0 | 17.0 ± 16.2 | 20.2 ± 20.6 | 24.6 ± 25.04 | ||
| Sig. | 0.694 | 0.438 | 0.768 | 0.999 | 0.612 | 0.106 | 0.463 | ||
| WBC (×103/µL) | Before | 4.81 ± 1.72 | 4.75 ± 1.37 | 4.67 ± 1.31 | 4.90 ± 1.45 | 5.3 ± 3.0 | 5.13 ± 1.80 | 4.54 ± 1.56 | F = 1.12 p = 0.996 * |
| After | 6.92 ± 2.21 | 6.78 ± 1.79 | 6.56 ± 1.97 | 6.64 ± 1.95 | 6.72 ± 2.83 | 6.91 ± 2.02 | 6.74 ± 2.37 | ||
| Sig. | 0.005 ** | 0.001 ** | 0.005 ** | 0.001 ** | 0.104 | 0.001 ** | 0.001 ** | ||
| CD Profile | Before vs. after Treatment (Mean ± S.D) | Post-Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (n = 11) | Group 2 (n = 15) | Group 3 (n = 13) | Group 4 (n = 18) | Group 5 (n = 12) | Group 6 (n = 15) | Group-7 (n = 13) | Sig. | ||
| CD3 | Before | 72.57 ± 5.57 | 73.98 ± 9.31 | 75.83 ± 3.0 | 73.85 ± 7.53 | 78.59 ± 4.47 | 75.66 ± 5.29 | 71.99 ± 8.50 | 0.461 |
| After | 72.15 ± 5.16 | 74.95 ± 8.23 | 76.02 ± 4.87 | 74.20 ± 7.44 | 76.93 ± 4.77 | 77.18 ± 5.59 | 73.78 ± 8.06 | ||
| Sig. | 0.657 | 0.609 | 0.861 | 0.777 | 0.117 | 0.638 | 0.279 | ||
| CD19 | Before | 9.54 ± 2.81 | 8.81 ± 3.83 | 9.11 ± 4.72 | 9.05 ± 4.01 | 10.38 ± 3.48 | 8.31 ± 2.89 | 6.89 ± 3.69 | 0.295 |
| After | 14.31 ± 3.37 * | 11.65 ± 4.67 * | 11.04 ± 4.34 * | 12.95 ± 5.04 * | 13.55 ± 4.78 * | 11.63 ± 3.84 * | 10.50 ± 4.38 * | ||
| Sig. | 0.010 | 0.001 | 0.039 | 0.000 | 0.003 | 0.005 | 0.005 | ||
| CD16+/CD56 | Before | 17.23 ± 4.42 | 16.47 ± 9.13 | 13.99 ± 3.69 | 16.11 ± 6.12 | 9.93 ± 4.08 | 14.12 ± 6.01 | 19.81 ± 9.36 | 0.234 |
| After | 12.44 ± 5.45 * | 12.09 ± 7.04 * | 11.71 ± 5.48 | 11.72 ± 4.36 * | 8.17 ± 3.76 | 10.19 ± 4.68 * | 14.48 ± 9.29 * | ||
| Sig. | 0.008 | 0.005 | 0.055 | 0.001 | 0.136 | 0.001 | 0.001 | ||
| CD3+/CD4+ | Before | 43.27 ± 6.01 | 41.75 ± 9.74 | 40.00 ± 7.24 | 42.67 ± 8.35 | 46.21 ± 8.33 | 47.12 ± 4.98 | 39.43 ± 8.33 | 0.136 |
| After | 44.45 ± 6.76 | 43.61 ± 9.43 | 38.17 ± 12.13 | 44.25 ± 7.07 | 45.49 ± 7.52 | 47.91 ± 5.49 | 42.08 ± 10.51 * | ||
| Sig. | 0.286 | 0.173 | 0.552 | 0.306 | 0.480 | 0.691 | 0.033 | ||
| CD3+/CD8+ | Before | 26.79 ± 4.87 | 30.49 ± 6.98 | 33.55 ± 7.89 | 28.24 ± 6.70 | 29.45 ± 9.89 | 29.35 ± 7.83 | 30.12 ± 7.77 | 0.340 |
| After | 25.15 ± 5.07 | 29.50 ± 6.43 | 31.74 ± 7.17 | 26.56 ± 8.42 | 29.34 ± 8.09 | 29.23 ± 7.53 | 29.61 ± 7.38 | ||
| Sig. | 0.062 | 0.233 | 0.173 | 0.528 | 0.814 | 0.865 | 0.552 | ||
| T-HELPER/T-SUPPRESSOR RATIO | Before | 1.68 ± 0.45 | 1.48 ± 0.65 | 1.30 ± 0.48 | 1.62 ± 0.60 | 1.41 ± 0.30 | 1.78 ± 0.76 | 1.43 ± 0.57 | 0.591 |
| After | 1.86 ± 0.55 | 1.59 ± 0.70 | 1.39 ± 0.46 | 1.52 ± 0.54 | 1.90 ± 1.56 | 1.78 ± 0.65 | 1.54 ± 0.59 | ||
| Sig. | 0.103 | 0.088 | 0.328 | 0.616 | 0.638 | 0.910 | 0.064 | ||
| CD3 Abs cnt | Before | 1534.58 ± 684.36 | 1495.31 ± 1052.90 | 1798.11 ± 596.49 | 1642.30 ± 809.47 | 1806.99 ± 1137.98 | 1398.58 ± 881.03 | 1272.97 ± 705.45 | 0.551 |
| After | 1913.36 ± 569.83 | 1888.60 ± 754.61 * | 1887.65 ± 645.65 | 1828.90 ± 607.53 | 2116.15 ± 756.48 | 1874.67 ± 788.32 * | 1517.86 ± 685.32 | ||
| Sig. | 0.248 | 0.023 | 0.552 | 0.170 | 0.117 | 0.003 | 0.196 | ||
| CD19 Abs cnt | Before | 212.07 ± 130.31 | 164.01 ± 120.10 | 250.80 ± 240.22 | 196.62 ± 128.40 | 246.87 ± 183.70 | 153.03 ± 108.16 | 105.46 ± 58.76 | 0.221 |
| After | 393.75 ± 192.27 * | 278.77 ± 144.68 * | 295.66 ± 219.53 | 344.79 ± 225.68 * | 381.60 ± 206.75 * | 295.46 ± 190.84 * | 212.17 ± 116.04 * | ||
| Sig. | 0.041 | 0.001 | 0.116 | 0.006 | 0.028 | 0.004 | 0.002 | ||
| CD16+ CD56 Abs cnt | Before | 345.33 ± 138.71 | 323.59 ± 296.55 | 329.54 ± 109.72 | 332.57 ± 163.42 | 209.33 ± 117.18 | 208.18 ± 102.09 | 301.24 ± 213.18 | 0.607 |
| After | 309.38 ± 108.03 | 290.89 ± 196.13 | 265.35 ± 87.81 | 290.14 ± 138.16 | 213.56 ± 105.62 | 223.77 ± 113.88 | 279.69 ± 233.50 | ||
| Sig. | 0.424 | 0.394 | 0.028 | 0.267 | 0.875 | 0.532 | 0.382 | ||
| CD3+/CD4+ Abs cnt | Before | 931.99 ± 479.65 | 728.31 ± 334.49 | 1017.77 ± 475.71 | 864.17 ± 303.56 | 1028.44 ± 578.31 | 846.13 ± 480.68 | 633.83 ± 328.46 | 0.468 |
| After | 1200.94 ± 468.37 | 1012.03 ± 333.43 * | 1042.84 ± 437.75 | 1115.23 ± 448.10 * | 1201.43 ± 323.73 | 1160.51 ± 485.67 * | 889.63 ± 424.33 * | ||
| Sig. | 0.248 | 0.002 | 0.972 | 0.018 | 0.084 | 0.005 | 0.019 | ||
| CD3+/CD8+ Abs cnt | Before | 548.44 ± 218.77 | 546.30 ± 297.28 | 796.24 ± 222.05 | 576.95 ± 244.41 | 701.23 ± 549.44 | 545.62 ± 408.56 | 450.82 ± 202.27 | 0.466 |
| After | 647.00 ± 144.35 | 690.14 ± 234.42 * | 769.13 ± 239.94 | 658.75 ± 158.52 | 820.91 ± 434.80 | 712.76 ± 384.16 * | 590.42 ± 289.93 * | ||
| Sig. | 0.213 | 0.012 | 0.422 | 0.170 | 0.099 | 0.006 | 0.033 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Abdulrahman, K.A.; Bamosa, A.O.; Bukhari, A.I.; Siddiqui, I.A.; Arafa, M.A.; Mohsin, A.A.; Althageel, M.F.; Aljuaeed, M.O.; Aldeailej, I.M.; Alrajeh, A.I.; et al. The Effect of Short Treatment with Nigella Sativa on Symptoms, the Cluster of Differentiation (CD) Profile, and Inflammatory Markers in Mild COVID-19 Patients: A Randomized, Double-Blind Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 11798. https://doi.org/10.3390/ijerph191811798
Bin Abdulrahman KA, Bamosa AO, Bukhari AI, Siddiqui IA, Arafa MA, Mohsin AA, Althageel MF, Aljuaeed MO, Aldeailej IM, Alrajeh AI, et al. The Effect of Short Treatment with Nigella Sativa on Symptoms, the Cluster of Differentiation (CD) Profile, and Inflammatory Markers in Mild COVID-19 Patients: A Randomized, Double-Blind Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(18):11798. https://doi.org/10.3390/ijerph191811798
Chicago/Turabian StyleBin Abdulrahman, Khalid A., Abdullah Omar Bamosa, Abdullah I. Bukhari, Intisar Ahmad Siddiqui, Mostafa A. Arafa, Ashfaq A. Mohsin, Mamdouh Faleh Althageel, Majed Owed Aljuaeed, Ibrahim Mohammed Aldeailej, Abdulaziz Ibrahim Alrajeh, and et al. 2022. "The Effect of Short Treatment with Nigella Sativa on Symptoms, the Cluster of Differentiation (CD) Profile, and Inflammatory Markers in Mild COVID-19 Patients: A Randomized, Double-Blind Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 18: 11798. https://doi.org/10.3390/ijerph191811798
APA StyleBin Abdulrahman, K. A., Bamosa, A. O., Bukhari, A. I., Siddiqui, I. A., Arafa, M. A., Mohsin, A. A., Althageel, M. F., Aljuaeed, M. O., Aldeailej, I. M., Alrajeh, A. I., Aldosari, K. M., Hawsawi, N. A., Zawbaee, K. I., & Alsurayea, S. M. (2022). The Effect of Short Treatment with Nigella Sativa on Symptoms, the Cluster of Differentiation (CD) Profile, and Inflammatory Markers in Mild COVID-19 Patients: A Randomized, Double-Blind Controlled Trial. International Journal of Environmental Research and Public Health, 19(18), 11798. https://doi.org/10.3390/ijerph191811798