Time-Resolved Kinetic Measurement of Microalgae Agglomeration for Screening of Polysaccharides-Based Coagulants/Flocculants
Abstract
:1. Introduction
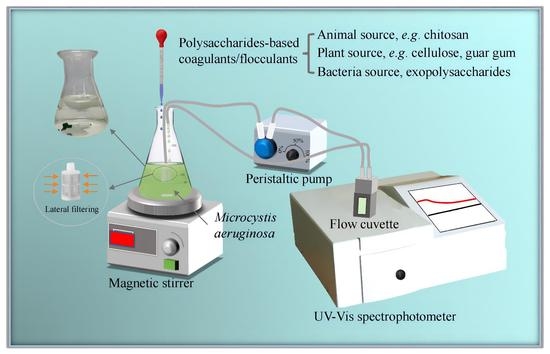
2. Materials and Methods
3. Results
3.1. Quantification of Microalgae by Visible Spectroscopy
3.2. TR Kinetic Coagulation Profiles and Model Fitting Curves
3.3. CFP Performance of Cationic Polysaccharides toward M. aeruginosa and C. vulgaris
3.4. Performance of BEPS as Flocculation Aids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water. 2019, 6, e1373. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Ye, X.; Ma, J.; Lv, M.; Lin, Z. Removal of Microcystis aeruginosa by natural pyrite-activated persulfate: Performance and the significance of iron species. Chem. Eng. J. 2022, 428, 132565. [Google Scholar] [CrossRef]
- Missaghi, S.; Hondzo, M.; Sun, C.; Guala, M. Influence of fluid motion on growth and vertical distribution of cyanobacterium Microcystis aeruginosa. Aquat. Ecol. 2016, 50, 639–652. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, D.; Guo, X.; He, Y.; Ong, C.N.; Xu, Y.; Pal, A. Removal of Microcystis aeruginosa using nano-Fe3O4 particles as a coagulant aid. Environ. Sci. Pollut. Res. Int. 2015, 22, 18731–18740. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.; Ward, A.; Ayoko, G.A.; Rosch, C.; Brown, R.; Rainey, T.J. Single-step dynamic dewatering of microalgae from dilute suspensions using flocculant assisted filtration. Microb. Cell Factories. 2020, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhao, Y.-X. Dosage and pH dependence of coagulation with polytitanium salts for the treatment of Microcystis aeruginosa-laden and Microcystis wesenbergii-laden surface water: The influence of basicity. J. Water Process. Eng. 2021, 39, 101726. [Google Scholar] [CrossRef]
- Matter, I.A.; Bui, V.K.H.; Jung, M.; Seo, J.Y.; Kim, Y.E.; Lee, Y.C.; Oh, Y.K. Flocculation harvesting techniques for microalgae: A review. Appl. Sci. 2019, 9, 3069. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, Y.; Jiang, X.; Wang, Y.; Li, H.; Wang, L.; Liang, W. The growth and physiological activity of Microcystis aeruginosa after flocculation using modified tannin. Int. Biodeterior. 2018, 133, 180–186. [Google Scholar] [CrossRef]
- Mucci, M.; Guedes, I.A.; Faassen, E.J.; Lurling, M. Chitosan as a coagulant to remove cyanobacteria can cause microcystin release. Toxins. 2020, 12, 711. [Google Scholar] [CrossRef]
- Li, N.J.; Lan, Q.; Wu, J.H.; Liu, J.; Zhang, X.H.; Zhang, F.; Yu, H.Q. Soluble microbial products from the white-rot fungus Phanerochaete chrysosporium as the bioflocculant for municipal wastewater treatment. Sci. Total Environ. 2021, 780, 146662. [Google Scholar] [CrossRef]
- Xu, L.; Huo, M.; Sun, C.; Cui, X.; Zhou, D.; Crittenden, J.C.; Yang, W. Bioresources inner-recycling between bioflocculation of Microcystis aeruginosa and its reutilization as a substrate for bioflocculant production. Sci. Rep. 2017, 7, 43784. [Google Scholar] [CrossRef] [Green Version]
- Nazari, M.T.; Rigueto, C.V.T.; Rempel, A.; Colla, L.M. Harvesting of Spirulina platensis using an eco-friendly fungal bioflocculant produced from agro-industrial by-products. Bioresour. Technol. 2021, 322, 124525. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Jiang, X.; Zhang, K.; Zheng, T.; Wang, H. First evidence of bioflocculant from Shinella albus with flocculation activity on harvesting of Chlorella vulgaris biomass. Bioresour. Technol. 2016, 218, 807–815. [Google Scholar] [CrossRef]
- Eldridge, R.J.; Hill, D.R.A.; Gladman, B.R. A comparative study of the coagulation behaviour of marine microalgae. J. Appl. Phycol. 2012, 24, 1667–1679. [Google Scholar] [CrossRef]
- Mehto, P.; Ankelo, M.; Hinkkanen, A.; Mikhailov, A.; Eriksson, J.E.; Spoof, L.; Meriluoto, J. A time-resolved fluoroimmunometric assay for the detection of microcystins, cyanobacterial peptide hepatotoxins. Toxicon 2001, 39, 831–836. [Google Scholar] [CrossRef]
- Yang, J.; Holbach, A.; Wilhelms, A.; Qin, Y.; Zheng, B.; Zou, H.; Qin, B.; Zhu, G.; Norra, S. Highly time-resolved analysis of seasonal water dynamics and algal kinetics based on in-situ multi-sensor-system monitoring data in Lake Taihu, China. Sci. Total. Environ. 2019, 660, 329–339. [Google Scholar] [CrossRef]
- Ho, K.-S.; Chan, W.-T. Time-resolved ICP-MS measurement for single-cell analysis and on-line cytometry. J. Anal. At. Spectrom. 2010, 25, 1114–1122. [Google Scholar] [CrossRef]
- Wang, K.; Ye, L. Structure and property of cationic hydroxyethyl cellulose. Polym. Plast. Technol. Eng. 2010, 49, 807–811. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Selvaraju, N.; Raj Mohan, B.; Muhammed Anzil, P.K.; Amith, K.D.; Ushakumary, E.R. Hydrous cerium oxide nanoparticles impregnated Enteromorpha sp. for the removal of hexavalent chromium from aqueous solutions. J. Environ. Eng. 2016, 142, C4015016. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of pigments from microalgae and cyanobacteria—A review on current methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Ding, N.; Li, C.; Wang, T.; Guo, M.; Mohsin, A.; Zhang, S. Evaluation of an enclosed air-lift photobioreactor (ALPBR) for biomass and lipid biosynthesis of microalgal cells grown under fluid-induced shear stress. Biotechnol. Biotechnol. Equip. 2020, 35, 139–149. [Google Scholar] [CrossRef]
- Li, L.; Chen, W.; Wang, Y.; Zhang, Y.; Chen, H. Effect of hydrodynamics on autoflocculation and gravity sedimentation of Chlorella vulgaris. J. Water Process. Eng. 2021, 43, 102259. [Google Scholar] [CrossRef]
- Liu, J.L.; Hsieh, H.J.; Eisenberg, B. Poisson-Fermi modeling of the ion exchange mechanism of the sodium/calcium exchanger. J. Phys. Chem. 2016, 120, 2658–2669. [Google Scholar] [CrossRef] [PubMed]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Kothari, R.; Pathak, V.V.; Pandey, A.; Ahmad, S.; Srivastava, C.; Tyagi, V.V. A novel method to harvest Chlorella sp. via low cost bioflocculant: Influence of temperature with kinetic and thermodynamic functions. Bioresour. Technol. 2017, 225, 84–89. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, Z.; Wang, W.; Zhou, Z.; Kong, Y.; Ma, J. Bio-flocculation of Microcystis aeruginosa by using fungal pellets of Aspergillus oryzae: Performance and mechanism. J. Hazard. Mater. 2022, 439, 129606. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Flocculation characteristics of anaerobic sludge driven-extracellular polymeric substance (EPS) extracted by different methods on microalgae harvesting for lipid utilization. Biochem. Eng. J. 2021, 167, 107898. [Google Scholar] [CrossRef]
- Ma, R.; Wang, B.; Chua, E.T.; Zhao, X.; Lu, K.; Ho, S.H.; Chen, J. Comprehensive utilization of marine microalgae for enhanced co-production of multiple compounds. Mar. Drugs 2020, 18, 467. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Tian, Y.; Zhou, X.; Wang, S.; Zhu, J.; Liu, C. An in situ extractive fermentation strategy for enhancing prodigiosin production from Serratia marcescens BWL1001 and its application to inhibiting the growth of Microcystis aeruginosa. Biochem. Eng. J. 2021, 166, 107836. [Google Scholar] [CrossRef]
- Fei, Y.A.N.G.; Wei, H.Y.; Li, X.Q.; Li, Y.H.; Li, X.B.; Yin, L.H.; Pu, Y.P. Isolation and characterization of an algicidal bacterium indigenous to Lake Taihu with a red pigment able to lyse Microcystis aeruginosa. Biomed. Environ. Sci. 2013, 26, 148–154. [Google Scholar]
- Liu, H.; Zhu, J.Y.; Chai, X.S. In situ, rapid, and temporally resolved measurements of cellulase adsorption onto lignocellulosic substrates by UV− Vis spectrophotometry. Langmuir 2011, 27, 272–278. [Google Scholar] [CrossRef]
- Udayan, A.; Sirohi, R.; Sreekumar, N.; Sang, B.I.; Sim, S.J. Mass cultivation and harvesting of microalgal biomass: Current trends and future perspectives. Bioresour. Technol. 2022, 344, 126406. [Google Scholar] [CrossRef]
- Izadpanah, M.; Gheshlaghi, R.; Mahdavi, M.A.; Elkamel, A. Effect of light spectrum on isolation of microalgae from urban wastewater and growth characteristics of subsequent cultivation of the isolated species. Algal Res. 2018, 29, 154–158. [Google Scholar] [CrossRef]




| Microalgae | PBCFs | Dosage (gCFs/gm) | ks (gCFs/gm/min) | qe (gm/gCFs) | ks·qe2 (gm·gCFs/min) | R2 |
|---|---|---|---|---|---|---|
| M. aeruginosa | CHEC | 0.07 | 3.23 | 0.437 | 0.60 | 0.9906 |
| CQA | 0.07 | 5.13 | 0.423 | 0.93 | 0.9902 | |
| CGG | 0.07 | 4.92 | 0.16 | 0.13 | 0.9897 | |
| S. platensis | CQA | 0.07 | 10.08 | 0.44 | 1.97 | 0.9895 |
| S. maxima | CQA | 0.07 | 11.11 | 0.43 | 2.64 | 0.9863 |
| C. vulgaris | CQA | 0.07 | 128.51 | 0.06 | 0.39 | 0.0989 |
| I. galbana | CQA | 0.07 | Not coagulated | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Jia, Y.; Gong, X.; Liu, H.; Sun, C. Time-Resolved Kinetic Measurement of Microalgae Agglomeration for Screening of Polysaccharides-Based Coagulants/Flocculants. Int. J. Environ. Res. Public Health 2022, 19, 14610. https://doi.org/10.3390/ijerph192114610
Zhou J, Jia Y, Gong X, Liu H, Sun C. Time-Resolved Kinetic Measurement of Microalgae Agglomeration for Screening of Polysaccharides-Based Coagulants/Flocculants. International Journal of Environmental Research and Public Health. 2022; 19(21):14610. https://doi.org/10.3390/ijerph192114610
Chicago/Turabian StyleZhou, Jinxia, Yunlu Jia, Xiaobei Gong, Hao Liu, and Chengwu Sun. 2022. "Time-Resolved Kinetic Measurement of Microalgae Agglomeration for Screening of Polysaccharides-Based Coagulants/Flocculants" International Journal of Environmental Research and Public Health 19, no. 21: 14610. https://doi.org/10.3390/ijerph192114610
APA StyleZhou, J., Jia, Y., Gong, X., Liu, H., & Sun, C. (2022). Time-Resolved Kinetic Measurement of Microalgae Agglomeration for Screening of Polysaccharides-Based Coagulants/Flocculants. International Journal of Environmental Research and Public Health, 19(21), 14610. https://doi.org/10.3390/ijerph192114610