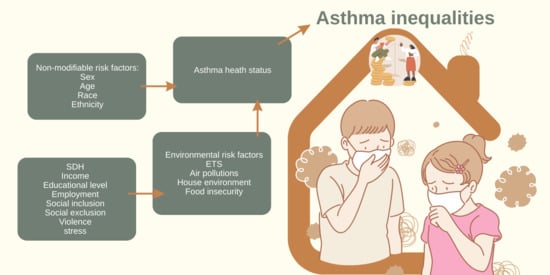
Social Inequalities: Do They Matter in Asthma, Bronchitis, and Respiratory Symptoms in Children?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Group
2.2. Eligibility Criteria
2.3. Research Tool
- High SES—higher or secondary education, both parents working, and good self-assessment of economic status;
- Middle SES—middle or lower than the middle level of education, at least one parent working, and average self-assessment of economic status;
- Low SES—primary or vocational level of education, unemployed, and low self-assessment of economic status
2.4. Statistical Analyses
3. Results
3.1. Descriptive Analysis
3.2. Multivariate Logistic Analysis
4. Discussion
4.1. This Work
4.2. Strengths and Limitations
4.3. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ISAAC | The International Study on Asthma and Allergies in Childhood |
| OR | odds ratio |
| SDH | social determinants of health |
| SES | socioeconomic status |
| WHO | World Health Organization |
References
- World Health Organization. Health Inequalities and Their Causes. 2018. Available online: https://www.who.int/news-room/facts-in-pictures/detail/health-inequities-and-their-causes (accessed on 20 September 2022).
- Sztompka, P. Socjologia; Wydawnictwo Znak: Kraków, Poland, 2003; pp. 333–355. [Google Scholar]
- Wilkinson, R.G.; Marmot, M.; World Health Organization; Regional Office for Europe. The Solid Facts: Social Determinants of Health; WHO Regional Office for Europe: Copenhagen, Denmark, 1998; Available online: https://apps.who.int/iris/handle/10665/108082 (accessed on 20 September 2022).
- Wypych-Ślusarska, A.; Niewiadomska, E.; Oleksiuk, K.; Krupa-Kotara, K.; Głogowska-Ligus, J.; Słowiński, J. Caesarean delivery and risk of childhood asthma development: Meta-analysis. Postepy Dermatologii i Alergologii 2021, 5, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, D.N.; Zierler, S.; Meersman, S.; Kim, H.K.; Caron, C.; Island, R. Race disparities in childhood asthma: Does where you live matter? J. Natl. Med. Assoc. 2006, 2, 239–247. [Google Scholar]
- Gold, D.R.; Wright, R. Population disparities in asthma. Annu. Rev. Public Health 2005, 26, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Redmond, C.; Akinoso-Imran, A.Q.; Heaney, L.G.; Sheikh, A.; Kee, F.; Busby, J. Socioeconomic disparities in asthma health care utilization, exacerbations, and mortality: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2022, 5, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Wypych-Ślusarska, A.; Grot, M.; Kujawińska, M.; Nigowski, M.; Krupa-Kotara, K.; Oleksiuk, K.; Głogowska-Ligus, J.; Grajek, M. Respiratory Symptoms, Allergies, and Environmental Exposures in Children with and without Asthma. Int. J. Environ. Res. Public Health 2022, 19, 11180. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: https://ginasthma.org/reports/ (accessed on 20 September 2022).
- Samoliński, B.; Raciborski, F.; Lipiec, A.; Tomaszewska, A.; Krzych-Fałta, E.; Samel-Kowalik, P.; Walkiewicz, A.; Lusawa, A.; Borowicz, J.; Komorowski, J.; et al. Epidemiology of Allergic Diseases in Poland (ECAP). Alergol. Pol. Pol. J. Allergol. 2014, 1, 10–18. [Google Scholar] [CrossRef]
- Forno, E.; Celedón, J.C. Health Disparities in Asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 1033–1043. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.; Murphy, A.; Stallberg, B.; Baxter, N.; Heaney, L.G. ‘SIMPLES’: A structured primary care approach to adults with difficult asthma. Prim. Care Respir. J. 2013, 22, 365–373. [Google Scholar] [CrossRef]
- Ścibor, M.; Balcerzak, B.; Malinowska-Cieślik, M. Czy środowisko może mieć wpływ na jakość życia pacjentów z astmą oskrzelową? Zdrowie Publiczne i Zarządzanie 2012, 10, 25–30. [Google Scholar] [CrossRef]
- MacNeill, S.J.; Sozanska, B.; Danielewicz, H.; Debinska, A.; Kosmeda, A.; Boznanski, A.; Illi, S.; Depner, M.; Strunz-Lehner, C.; Waser, M.; et al. Asthma and allergies: Is the farming environment (still) protective in Poland? The GABRIEL Advanced Studies. Allergy 2013, 68, 771–779. [Google Scholar] [CrossRef]
- Cianciara, D. Causes and causes of the causes of health inequalities. Hyg. Pub. Health. 2015, 3, 435–440. [Google Scholar]
- Urząd Statystyczny w Katowicach. Komunikat o Sytuacji Społeczno-Gospodarczej Województwa Śląskiego w Listopadzie 2020 r. 30.12.2020. November 2020. Available online: https://katowice.stat.gov.pl/opracowania-biezace/komunikaty-i-biuletyny/inne-opracowania/komunikat-o-sytuacji-spoleczno-gospodarczej-wojewodztwa-slaskiego-w-listopadzie-2020-r-,2,108.html (accessed on 16 October 2022).
- Główny Urząd Statystyczny. Ochrona środowiska 2021, Warszawa 2021. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2021,1,22.html (accessed on 16 October 2022).
- Mulry, M. Coverage error. In Encyclopedia of Survey Research Methods; Lavrakas, P.J., Ed.; Sage Publications, Inc.: Los Angeles, CA, USA, 2008; pp. 162–166. [Google Scholar] [CrossRef]
- Ryan, D.; Sabroe, I. Identifying and addressing health inequalities in asthma care. Eur. Respir J. 2021, 6, 2101829. [Google Scholar] [CrossRef] [PubMed]
- Leonard, T.; Hughes, A.E.; Pruitt, S.L. Understanding how low-socioeconomic status households cope with health shocks: An analysis of multi-sector linked data. Ann. Am. Acad. Pol. Soc. Sci. 2017, 1, 125–145. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, D.K.; Dell, S.D.; Guttmann, A.; Shariff, S.Z.; Liu, K.; To, T. Trends in the age of diagnosis of childhood asthma. J. Allergy Clin. Immunol. 2014, 5, 1057–1062.e5. [Google Scholar] [CrossRef]
- Marmot, M. Inequalities in asthma mortality: A specific case of a general issue of health inequalities. Thorax 2018, 8, 704–705. [Google Scholar] [CrossRef]
- Williams, D.R.; Sternthal, M.; Wright, R.J. Social determinants: Taking the social context of asthma seriously. Pediatrics 2009, 123 (Suppl. 3), S174–S184. [Google Scholar] [CrossRef] [Green Version]
- Grand, T.; Croce, E.; Matusi, E.C. Asthma and the social determinants of health. Ann. Allergy Asthma Immunol. 2022, 1, 5–11. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V. Chronic cough due to asthma: ACCP evidence-based clinical practice guidelines. Chest 2006, 1, 75S–79S. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.J.; Song, W.J.; Kang, S.H. The disease burden and quality of life of chronic cough patients in South Korea and Taiwan. World Allergy Organ J. 2022, 9, 100681. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Kumar, P.; Srivastava, S.; Muhammad, T. Understanding socio-economic inequalities in the prevalence of asthma in India: An evidence from national sample survey 2017-18. BMC Pulm. Med. 2021, 1, 72. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.A.; Morgan, W.J.; Halonen, M.; Wright, A.L.; Martinez, F.D. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: A longitudinal birth-cohort study. Lancet 2008, 20, 1058–1064. [Google Scholar] [CrossRef]
- Håkansson, K.E.J.; Backer, V.; Ulrik, C.S. Socioeconomic biases in asthma control and specialist referral of possible severe asthma. Eur. Respir. J. 2021, 6, 2100741. [Google Scholar] [CrossRef] [PubMed]
- Asaad, C.; Ghogho, M. AsthmaKGxE: An asthma-environment interaction knowledge graph leveraging public databases and scientific literature. Comput. Biol. Med. 2022, 148, 105933. [Google Scholar] [CrossRef] [PubMed]
- Molnár, D.; Gálffy, G.; Horváth, A.; Tomisa, G.; Katona, G.; Hirschberg, A.; Mezei, G.; Sultész, M. Prevalence of Asthma and Its Associating Environmental Factors among 6-12-Year-Old Schoolchildren in a Metropolitan Environment-A Cross-Sectional, Questionnaire-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 13403. [Google Scholar] [CrossRef]
- Jaakkola, J.J.; Hwang, B.F.; Jaakkola, N. Home dampness and molds, parental atopy, and asthma in childhood: A six-year population-based cohort study. Environ. Health Perspect. 2005, 3, 357–361. [Google Scholar] [CrossRef]
- Apichainan, N.; Norkaew, S.; Taneepanichskul, N. Residential environment in relation to self-report of respiratory and asthma symptoms among primary school children in a high-polluted urban area. Sci. Rep. 2022, 1, 2946. [Google Scholar] [CrossRef]
- Burke, H.; Leonardi-Bee, J.; Hashim, A.; Pine-Abata, H.; Chen, Y.; Cook, D.G.; Britton, J.R.; McKeever, T.M. Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 2012, 129, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Bentouhami, H.; Casas, L.; Weyler, J. The Association between the Occurrence of Asthma and Antecedents of Exposure to Environmental Tobacco Smoke in the Previous Year in Children: An Incidence-Density Study. Int. J. Environ. Res. Public Health. 2022, 19, 2888. [Google Scholar] [CrossRef]
- Mahabee-Gittens, E.M.; Merianos, A.L.; Fulkerson, P.C.; Stone, L.; Matt, G.E. The Association of Environmental Tobacco Smoke Exposure and Inflammatory Markers in Hospitalized Children. Int. J. Environ. Res. Public Health 2019, 16, 4625. [Google Scholar] [CrossRef] [Green Version]
- Shahunja, K.M.; Sly, P.D.; Begum, T.; Biswas, T.; Mamun, A. Family, neighborhood and psychosocial environmental factors and their associations with asthma in Australia: A systematic review and Meta-analysis. J. Asthma 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Sio, Y.Y.; Chew, F.T. Risk factors of asthma in the Asian population: A systematic review and meta-analysis. J. Physiol. Anthropol. 2021, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Rönmark, E.; Andersson, C.; Nyström, L.; Forsberg, B.; Järvholm, B.; Lundbäck, B. Obesity increases the risk of incident asthma among adults. Eur. Respir. J. 2005, 25, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penman-Aguilar, A.; Talih, M.; Huang, D.; Moonesinghe, R.; Bouye, K.; Beckles, G. Measurement of Health Disparities, Health Inequities, and Social Determinants of Health to Support the Advancement of Health Equity. J. Public Health Manag. Pract. 2016, 1, S33–S42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakes, J.M.; Rossi, P.M. The measurement of SES in health research: Current practice and steps toward a new approach. Soc. Sci. Med. 2003, 4, 769–784. [Google Scholar] [CrossRef]
- Muhajarine, N.; McRae, D.; Soltanifar, M. Aboriginal Status and Neighbor hood Income Inequality Moderate the Relationship between School Absenteeism and Early Childhood Development. Int. J. Environ. Res. Public Health 2019, 16, 1347. [Google Scholar] [CrossRef]

| Social and Demographic Variable | Total n (%) | Asthma n (%) | p * | Bronchitis n (%) | p * | Wheezing Attacks at Nights n (%) | p * | Wheezy in the Last 12 Months n (%) | p * | Chronic Cough n (%) | p * | Dyspnea in the Last 12 Months n (%) | p * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||||||
| male | 1571 (49.0) | 180 (11.4) | <0.001 | 288 (18.3) | 0.03 | 102 (6.5) | 0.07 | 165 (11.0) | 0.01 | 159 (10.1) | 0.2 | 90 (5.73) | 0.009 |
| female | 1664 (54.0) | 118 (7.1) | 242 (14.5) | 84 (5.0) | 133 (8.0) | 147 (8.8) | 63 (3.79) | ||||||
| Mother’s education ** | |||||||||||||
| low *** | 527 (16.4) | 47 (8.9) | 0.2 | 84 (16.0) | 0.9 | 29 (5.5) | 0.8 | 51 (10.0) | 0.6 | 64 (12.1) | 0.006 | 24 (4.5) | 0.1 |
| middle | 1238 (38.6) | 127 (10.3) | 200 (16.1) | 75 (6.1) | 120 (10.0) | 126 (10.2) | 69 (5.6) | ||||||
| high | 1441 (45.0) | 121 (8.4) | 241 (16.7) | 80 (5.6) | 124 (9.0) | 112 (7.8) | 57 (4.0) | ||||||
| Father’s education ** | |||||||||||||
| low | 901 (30.0) | 88 (9.8) | 0.4 | 150 (16.6) | 0.9 | 60 (6.7) | 0.2 | 92 (10.0) | 0.1 | 101 (11.2) | 0.01 | 50 (5.5) | 0.3 |
| middle | 1100 (36.6) | 104 (9.4) | 175 (15.9) | 53 (4.8) | 86 (8.0) | 93 (8.4) | 46 (4.2) | ||||||
| high | 1005 (33.4) | 81 (8.0) | 165 (16.4) | 60 (6.0) | 98 (10.0) | 75 (7.5) | 43 (4.3) | ||||||
| Mother’s work activity | |||||||||||||
| working | 2479 (76.6) | 219 (8.8 | 0.2 | 393 (15.8) | 0.1 | 138 (5.6) | 0.4 | 220 (9.0) | 0.2 | 215 (8.7) | 0.005 | 113 (4.6) | 0.5 |
| non-working | 755 (33.4) | 79 (10.5) | 137 (18.1) | 48 (6.4) | 78 (10.0) | 91 (12.0) | 49 (5.2) | ||||||
| Father’s work activity | |||||||||||||
| working | 2909 (90.0) | 262 (9.0) | 0.2 | 477 (16.4) | 0.9 | 165 (5.7) | 0.5 | 266 (9.1) | 0.6 | 256 (8.8) | <0.001 | 131 (4.5) | 0.06 |
| non-working | 325 (10.0) | 36 (11.1) | 53 (16.3) | 21 (6.5) | 62 (9.8) | 50 (15.4) | 22 (6.8) | ||||||
| ETS | |||||||||||||
| yes | 1202 (37.2) | 125 (10.4) | 0.7 | 217 (18.0) | 0.04 | 72 (6.0) | 0.6 | 118 (9.8) | 0.4 | 143 (11.9) | <0.001 | 70 (5.8) | 0.02 |
| no | 2029 (62.8) | 173 (8.5) | 312 (15.4) | 114 (5.6) | 180 (8.9) | 163 (8.0) | 83 (4.1) | ||||||
| Type of heating | |||||||||||||
| central heating **** | 2759 (85.9) | 241 (8.7) | 0.02 | 445 (16.1) | 0.5 | 155 (5.6) | 0.7 | 239 (8.7) | 0.05 | 250 (9.1) | 0.1 | 127 (4.6) | 0.6 |
| coal burning | 453 (14.1) | 54 (11.9) | 79 (17.4) | 27 (6.0) | 52 (11.5) | 52 (11.5) | 23 (5.1) | ||||||
| Presence of mold | |||||||||||||
| yes | 782 (24.3) | 94 (12.0) | 0.001 | 156 (19.9) | 0.002 | 56 (7.2) | 0.05 | 97 (12.4) | <0.001 | 110 (14.0) | <0.001 | 45 (5.7) | 0.1 |
| no | 2440 (75.7) | 203 (8.3) | 372 (15.2) | 129 (5.3) | 199 (8.2) | 193 (7.9) | 107 (4.4) | ||||||
| Economic situation (self-assessment) | |||||||||||||
| good | 1998 (63.5) | 176 (8.8) | 0.5 | 327 (16.4) | 0.6 | 112 (5.6) | 0.3 | 188 (9.4) | 0.2 | 180 (9.0) | 0.01 | 90 (4.5) | 0.4 |
| average | 1066 (33.9) | 106 (9.9) | 176 (16.5) | 60 (5.6) | 89 (8.4) | 104 (9.8) | 52 (4.9) | ||||||
| bad | 82 (2.6) | 8 (9.8) | 10 (12.3) | 8 (9.8) | 11 (13.4) | 15 (18.3) | 6 (7.3) | ||||||
| SES | |||||||||||||
| high | 1932 (66.3) | 167 (8.6) | 0.2 | 303 (15.9) | 0.5 | 105 (5.4) | 0.5 | 171 (8.8) | 0.05 | 168 (8.7) | 0.04 | 90 (4.5) | 0.5 |
| middle | 462 (15.9) | 49 (10.6) | 75 (16.2) | 24 (5.2) | 35 (7.6) | 37 (8.0) | 52 (5.0) | ||||||
| low | 518 (17.8) | 55 (10.6) | 94 (18.1) | 35 (6.8) | 61 (11.8) | 62 (11.0) | 6 (7.3) | ||||||
| Age [years] | X ± SD | Asthma X ± SD | p * | Bronchitis X ± SD | p * | Wheezing attacks at nights X ± SD | p * | Wheezy in the last 12 months X ± SD | p * | Chronic cough X ± SD | p * | Dyspnea in the last 12 months X ± SD | p * |
| no | 10.6 ± 2.2 | 10.6 ± 2.2 | 0.5 | 10.7 ± 2.2 | 0.001 | 10.6 ± 2.2 | 0.002 | 10.7 ± 2.2 | 0.2 | 10.7 ± 2.2 | 0.1 | 10.6 ± 2.2 | 0.2 |
| yes ** | 10.6 ± 2.2 | 10.3 ± 2.1 | 10.1 ± 2.1 | 10.3 ± 2.2 | 10.3 ± 2.2 | 10.4 ± 2.2 | |||||||
| Health Problem | Determinants of Asthma, Bronchitis, and Respiratory Symptoms (* Reference Group) | OR (95%CI) Sex- and/or Age-Adjusted | p-Value |
|---|---|---|---|
| Asthma | Sex (male/female *) | 1.7 (1.33–2.16) | <0.001 |
| Presence of mould in apartments (yes/no *) | 1.5 (1.17–1.96) | 0.002 | |
| Type of heating (coal/clean *) | 1.4 (1.03–1.92) | 0.03 | |
| Bronchitis | Sex (male/female *) | 1.3 (1.10–1.59) | 0.003 |
| Presence of mold in apartments (yes/no *) | 1.4 (1.13–1.72) | 0.002 | |
| ETS (yes/no *) | 1.2 (0.99–1.46) | 0.05 | |
| Age [years] | 0.9 (1.09–1.59) | 0.001 | |
| Wheezing attacks at nights | Presence of mold in apartments (yes/no *) | 1.4 (1.01–1.93) | 0.04 |
| Age [years] | 0.9 (0.82–0.95) | 0.002 | |
| Wheezy in the last 12 months | Sex (male/female *) | 1.4 (1.06–1.71) | 0.01 |
| Presence of mold in apartments (yes/no *) | 1.6 (1.24–2.08) | <0.001 | |
| SES (low/middle/high *) | 0.9 (0.59–1.26)/ 1.4 (1.06–1.97) | 0.08/ 0.008 | |
| Chronic cough | Mother’s education (low */middle/high) | 0.8 (0.58–1.11)/ 0.6 (0.41–0.79) | 0.6/ <0.001 |
| Father’s education (low */middle/high) | 0.7 (0.52–0.94)/ 0.6 (0.43–0.82) | 0.5/ 0.02 | |
| Mother’s work activity (working/not working *) | 0.7 (0.54–0.91) | 0.007 | |
| Father’s work activity (working/non-working *) | 0.5 (0.37–0.71) | <0.001 | |
| ETS (yes/no *) | 1.5 (1.22–1.96) | <0.001 | |
| Presence of mould (yes/no *) | 1.9 (1.49–2.46) | <0.001 | |
| Self-assessment of economic situation (bad */average/good) | 0.4 (0.24–0.78) 0.5 (0.26–0.87) | 0.007/ 0.07 | |
| SES (low/middle/high *) | 0.9 (0.64–1.34)/ 1.5 (1.09–2.03) | 0.14/ 0.009 | |
| Dyspnea in the last 12 months | Sex (male/female *) | 1.5 (1.11–2.14) | 0.01 |
| ETS (yes/no *) | 1.4 (1.04–2.00) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wypych-Ślusarska, A.; Krupa-Kotara, K.; Niewiadomska, E. Social Inequalities: Do They Matter in Asthma, Bronchitis, and Respiratory Symptoms in Children? Int. J. Environ. Res. Public Health 2022, 19, 15366. https://doi.org/10.3390/ijerph192215366
Wypych-Ślusarska A, Krupa-Kotara K, Niewiadomska E. Social Inequalities: Do They Matter in Asthma, Bronchitis, and Respiratory Symptoms in Children? International Journal of Environmental Research and Public Health. 2022; 19(22):15366. https://doi.org/10.3390/ijerph192215366
Chicago/Turabian StyleWypych-Ślusarska, Agata, Karolina Krupa-Kotara, and Ewa Niewiadomska. 2022. "Social Inequalities: Do They Matter in Asthma, Bronchitis, and Respiratory Symptoms in Children?" International Journal of Environmental Research and Public Health 19, no. 22: 15366. https://doi.org/10.3390/ijerph192215366
APA StyleWypych-Ślusarska, A., Krupa-Kotara, K., & Niewiadomska, E. (2022). Social Inequalities: Do They Matter in Asthma, Bronchitis, and Respiratory Symptoms in Children? International Journal of Environmental Research and Public Health, 19(22), 15366. https://doi.org/10.3390/ijerph192215366