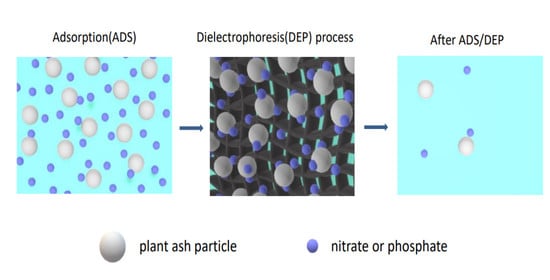
Highly Efficient Removal of Nitrate and Phosphate to Control Eutrophication by the Dielectrophoresis-Assisted Adsorption Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Experiments
2.3. Dielectrophoresis Experiments
2.4. Characterization of Adsorbents
3. Results and Discussion
3.1. Screening Test of Adsorbents
3.2. Optimization of the Adsorbent Pretreatment Time
3.3. Optimization of Adsorbent Dosage
3.4. Effect of the DEP Process
3.5. Effect of the Flow Rate
3.6. Optimization of the Voltage
3.7. Analysis of Plant Ash Particles by SEM and EDX
3.8. Characterization of Zeta Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Wu, Z.; Yu, W.; Lu, Y. Formation, harmfulness, prevention, control and treatment of waters eutrophication. Environ. Sci. Technol. 1999, 2, 12–16. [Google Scholar]
- Zhou, B.; Cai, X.; Wang, S.; Yang, X. Analysis of the Causes of Cyanobacteria Bloom: A Review. J. Resour. Ecol. 2020, 11, 405–413. [Google Scholar]
- Han, J.; Ro, H.M.; Cho, K.H.; Kim, K.W. Fluxes of nutrients and trace metals across the sediment-water interface controlled by sediment-capping agents: Bentonite and sand. Environ. Monit. Assess. 2016, 188, 566. [Google Scholar] [CrossRef]
- Xu, H.; Yang, G.; Zhou, J.; Qin, B.; Zhang, G.; Zou, H.; Hu, X. Effect of nitrogen and phosphorus concentration on colony growth of Microcystis flosaquae in Lake Taihu. J. Lake Sci. 2014, 2, 213–220. [Google Scholar]
- Smith, V.H. Low Nitrogen to Phosphorus Ratios Favor Dominance by Blue-Green Algae in Lake Phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Xi, B.; Xu, Q.; Gao, R.; Lu, Y.; Huang, J.; Liu, H. Application of stable isotope on nitrate pollution researches of surface water. J. Lake Sci. 2013, 5, 617–627. [Google Scholar]
- Moshoeshoe, M.N.; Obuseng, V. Simultaneous determination of nitrate, nitrite and phosphate in environmental samples by high performance liquid chromatography with UV detection. S. Afr. J. Chem. 2018, 71, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Guo, J.; Li, B. Form of Phosphorus in Low Concentration Phosphorus Removal Process. Mod. Chem. Res. 2020, 13, 33–35. [Google Scholar]
- Zhang, X.; Qiu, G. Causes of excessive use of chemical fertilizer and its impacts on China′s water environment security. South-to-North Water Transf. Water Sci. Technol. 2019, 4, 104–114. [Google Scholar]
- Wang, H.; Zhang, G.; Wu, F.; Ming, J.; Min, R.; Yan, W.; Cheng, J. Study on enhanced phosphorus removal effect of improved multi-stage A/O on low C/N domestic sewage. Chem. Ind. Eng. Prog. 2020, S2, 379–384. [Google Scholar]
- Sun, Y.; Zhang, X.; Li, D. Study on the Control of Nitrogen and Phosphorus Pollution in Taihu Food Industry—Take Beer Industry as an Example. Pollut. Control Technol. 2019, 6, 44–47. [Google Scholar]
- Lu, W.; Yao, X.; Zhang, B.; Gao, G.; Shao, K. Temporal-spatial Distribution of Nitrogen and Phosphorus Nutrients in Lake Taihu Based on Geostatistical Analysis. Environ. Sci. 2019, 2, 590–602. [Google Scholar]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, W.; Zhu, S.; Liu, F.; Qin, L.; Xu, C.; Wang, Z. Biological nitrogen and phosphorus removal by a phosphorus-accumulating bacteria Acinetobacter sp. strain C-13 with the ability of heterotrophic nitrification–aerobic denitrification. Bioresour. Technol. 2021, 322, 124507. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, F.; Ren, W. Kinetics of nitrate removal in groundwater using green synthesized nanoscale zero valent iron-nickel. Environ. Eng. 2018, 7, 71–76. [Google Scholar]
- Zhang, P.; Zhang, L.; Chen, S.; Zhu, J. Optimization and Validation of Electrochemical Technology for Simultaneous Nitrogen Removal in Aquaculture by Using Response Surface Methodology. Prog. Fish. Sci. 2020, 1, 66–74. [Google Scholar]
- Luo, Z.; Peng, J.; Wang, D.; Yang, J. Recovery of phosphate from piggery biogas slurry by ultrasonication, aeration and addition of MgO desulfurization waste residue. J. Clean. Prod. 2019, 211, 865–873. [Google Scholar] [CrossRef]
- Zhang, J. Study on the Effect of Calcium Peroxide Method on Sewage Treatment in Black Odor River. Jilin Water Resour. 2019, 8, 40–42+46. [Google Scholar]
- Huang, G.; Liu, F.; Qin, X.; Xin, X.; Jin, A. Remediation of nitrate-contaminated groundwater by the combination of iron and activated carbon. Technol. Water Treat. 2010, 5, 70–73+77. [Google Scholar]
- Yu, J.; Zhou, Y.; Yan, C.; Ma, H.; Wang, M.; Pu, S. Experimental research on the removal of nitrate from groundwater by a strain of hydrogen autotrophic denitrification bacterium. Ind. Water Treat. 2018, 10, 29–33. [Google Scholar]
- Fu, G.; Zhao, Y.; Zhou, S.; Chen, C.; Zhong, Y.; Xu, Y. Efficient removal of nitrogen and phosphorus in aqueous solutions using modified water treatment residuals–sodium alginate beads. Environ. Sci. Pollut. Res. 2021, 28, 46233–46246. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Daniel, H.; Michael, J.H.; Stuart, I. Dielectrophoresis: Developments and applications from 2010 to 2020. Electrophoresis 2020, 42, 539–564. [Google Scholar]
- Hu, J.; Chen, H.; Lan, B.; Geng, J.; Li, H.; Xing, X. A dielectrophoresis-assisted adsorption approach significantly facilitates the removal of cadmium species from wastewater. Environ. Sci. Water Res. Technol. 2015, 1, 199–203. [Google Scholar] [CrossRef]
- Liu, D.; Cui, C.; Wu, Y.; Chen, H.; Geng, J.; Xia, J. Highly efficient removal of ammonia nitrogen from wastewater by dielectrophoresis-enhanced adsorption. PeerJ 2018, 6, e5001. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Cui, C.; Chen, H.; Wang, Y.; Geng, J.; Wu, Y. Efficient removal of arsenic from water by dielectrophoresis-assisted adsorption. Water Supply 2019, 4, 1066–1072. [Google Scholar] [CrossRef]
- Jin, Q.; Cui, C.; Chen, H.; Wu, J.; Hu, J.; Xing, X.; Geng, J.; Wu, Y. Effective removal of Cd2+ and Pb2+ pollutants from wastewater by dielectrophoresis-assisted adsorption. Front. Environ. Sci. Eng. 2019, 13, 16. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Jiang, R. Research advances and prospects of phosphorus recovery from wastewater by biochar adsorption. China Sci. 2015, 3, 309–315. [Google Scholar]
- Li, J.; Wei, P.; Li, B.; Guo, J.; Li, J.; Yang, B.; Song, J. Nitrate Removal from Water by Activated Carbon Derived from Wheat Straw with FeCl3 Activation. J. Ecol. Rur. Environ. 2021, 37, 224–233. [Google Scholar]
- Gao, X.; Liu, P.; Li, A.; Song, H.; Li, Y.; Liu, Y.; Zhan, S. Research on removal of phosphate from water by electroadsorption technology. Acta Sci. Circumst. 2020, 40, 155–160. [Google Scholar]
- Pohl, H.A.; Crane, J.S. Dielectrophoretic force: A comparison of theory and experiment. J. Biol. Phys. 1978, 6, 133–160. [Google Scholar] [CrossRef]
- Tarkodjiel, M.; Mbaiguinam, M.; Ngaram, N.; Mahmout, Y.; Allaramadji, N. Elemental Composition of Vegetable Salts from Ash of Four Common Plants Species from Chad. Int. J. Pharm. 2012, 8, 582–585. [Google Scholar]
- Chang, L.; Yu, K.; Li, Y. The Study of the Adsorption of Methylene Blue by Plant Ash. Sichuan Environ. 2014, 33, 14–17. [Google Scholar]
- Mao, S.; Guo, X.; Zhou, Y.; Liu, Y.; Yang, Y.; Guo, G. Sorption kinetics and adsorption/desorption of tetraachlorobiphenyl onto plant ash. Environ. Sci. Technol. 2013, 36, 42–46. [Google Scholar]
- Chen, H.; Huang, H.; Zhu, Y.; Li, D. Factors impacting on the efficiency of dielectrophoresis treating of diesel fuel. Pet. Process. Petrochem. 2009, 7, 51–54. [Google Scholar]
- Yin, M.; Xiang, Y.; Si, Y.; Chen, T. Adsorption of Several Adsorbents onto Atrazine and Electrokinetic Properties. Soils 2012, 1, 118–125. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jin, Q.; Liang, Y.; Geng, J.; Xia, J.; Chen, H.; Yun, M. Highly Efficient Removal of Nitrate and Phosphate to Control Eutrophication by the Dielectrophoresis-Assisted Adsorption Method. Int. J. Environ. Res. Public Health 2022, 19, 1890. https://doi.org/10.3390/ijerph19031890
Li J, Jin Q, Liang Y, Geng J, Xia J, Chen H, Yun M. Highly Efficient Removal of Nitrate and Phosphate to Control Eutrophication by the Dielectrophoresis-Assisted Adsorption Method. International Journal of Environmental Research and Public Health. 2022; 19(3):1890. https://doi.org/10.3390/ijerph19031890
Chicago/Turabian StyleLi, Jiaxi, Qinghao Jin, Yuran Liang, Junfeng Geng, Jianxin Xia, Huiying Chen, and Miaoying Yun. 2022. "Highly Efficient Removal of Nitrate and Phosphate to Control Eutrophication by the Dielectrophoresis-Assisted Adsorption Method" International Journal of Environmental Research and Public Health 19, no. 3: 1890. https://doi.org/10.3390/ijerph19031890
APA StyleLi, J., Jin, Q., Liang, Y., Geng, J., Xia, J., Chen, H., & Yun, M. (2022). Highly Efficient Removal of Nitrate and Phosphate to Control Eutrophication by the Dielectrophoresis-Assisted Adsorption Method. International Journal of Environmental Research and Public Health, 19(3), 1890. https://doi.org/10.3390/ijerph19031890