Wastewater Monitoring for Infectious Disease: Intentional Relationships between Academia, the Private Sector, and Local Health Departments for Public Health Preparedness
Abstract
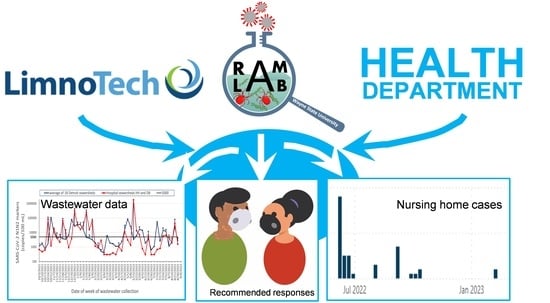
:1. Introduction
2. Development of the Monitoring Program
2.1. Site Selection and Collection
2.2. Site Categories
- (a)
- Small sewersheds with a focus on congregate facilities (LTCFs) in residential neighborhoods with at-risk populations;
- (b)
- Sewersheds that receive wastewater from university housing (dormitories);
- (c)
- Hospital center sewersheds;
- (d)
- “ZIP Code-wide” sewersheds with multiple neighborhoods and businesses.
2.2.1. Congregate Facilities with At-Risk Populations (LTCFs)
2.2.2. Congregate Facilities: University Housing (Dormitories)
2.2.3. Hospital Center Sewersheds
2.2.4. “ZIP Code” Sewersheds
2.2.5. Potential Future Collection Sites
3. Adoption and Improvement of Laboratory Analysis
4. Communication and Public Health Actions
4.1. Data Presentation and Reporting
4.2. Epidemiology and Data Response
- Focused, increased testing within sampled populations;
- Targeted vaccination campaigns or enhancement of capacity for existing programs;
- Encouragement of protective actions and cultural practices (social distancing, masking, hand-washing, etc.);
- Communication of potential higher risk to affected populations;
- Related resource planning in anticipation of a potential new outbreak;
- Investigations of clinical infection data at or near sites from which high marker levels have been reported.
4.3. Public Communication, Education, and Outreach
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, J.L.; Haidar, G.; Mellors, J.W. COVID-19: Challenges of viral variants. Annu. Rev. Med. 2023, 74, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Rai, C.I.; Chen, Y.C. Challenges and recent advancements in COVID-19 vaccines. Microorganisms 2023, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Kim, L.; Lu, M.; Nagoshi, K.; Namchuk, M.N. A review of clinical efficacy data supporting emergency use authorization for COVID-19 therapeutics and lessons for future pandemics. CTS-Clin. Transl. Sci. 2022, 15, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- D'Heysselaer, S.D.; Parisi, G.; Lisson, M.; Bruyere, O.; Donneau, A.F.; Fontaine, S.; Gillet, L.; Bureau, F.; Darcis, G.; Thiry, E.; et al. Systematic review of the key factors influencing the indoor airborne spread of SARS-CoV-2. Pathogens 2023, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Whitsel, L.P.; Ajenikoko, F.; Chase, P.J.; Johnson, J.; McSwain, B.; Phelps, M.; Radcliffe, R.; Faghy, M.A. Public policy for healthy living: How COVID-19 has changed the landscape. Prog. Cardiovasc. Dis. 2023, 76, 49–56. [Google Scholar] [CrossRef]
- Parmet, W.E.; Khalik, F. Judicial review of public health powers since the start of the COVID-19 pandemic: Trends and implications. Am. J. Public Health 2023, 113, 280–287. [Google Scholar] [CrossRef]
- Relihan, D.P.; Holman, E.A.; Garfin, D.R.; Ditto, P.H.; Silver, R.C. Politicization of a pathogen: A prospective longitudinal study of COVID-19 responses in a nationally representative US sample. Political Psychol. 2023, 1–44. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Fitzpatrick, M.A.; Bowen, S.A. Factors related to compliance with CDC COVID-19 guidelines: Media use, partisan identity, science knowledge, and risk assessment. West. J. Commun. 2023. [Google Scholar] [CrossRef]
- Pan, X.; Chen, D.; Xia, Y.; Wu, X.; Li, T.; Ou, X.; Zhou, L.; Liu, J. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Ahmed, W.; Bertsch, P.M.; Angel, N.; Bibby, K.; Bivins, A.; Dierens, L.; Edson, J.; Ehret, J.; Gyawali, P.; Hamilton, K.; et al. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: A surveillance tool for assessing the presence of COVID-19 infected travelers. J. Travel Med. 2020, 27, taaa116. [Google Scholar] [CrossRef]
- Galani, A.; Aalizadeh, R.; Kostakis, M.; Markou, A.; Alygizakis, N.; Lytras, T.; Adamopoulos, P.G.; Peccia, J.; Thompson, D.C.; Kontou, A.; et al. SARS-CoV-2 wastewater surveillance data can predict hospitalizations and ICU admissions. Sci. Total Environ. 2022, 804, 150151. [Google Scholar] [CrossRef] [PubMed]
- Panchal, D.; Prakash, O.; Bobde, P.; Pal, S. SARS-CoV-2: Sewage surveillance as an early warning system and challenges in developing countries. Environ. Sci. Pollut. Res. 2021, 28, 22221–22240. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Zulli, A.; Brackney, D.E.; Grubaugh, N.D.; Kaplan, E.H.; Casanovas-Massana, A.; Ko, A.I.; Malik, A.A.; Wang, D.; Wang, M.; et al. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. medRxiv 2020. [Google Scholar] [CrossRef]
- Prado, T.; Fumian, T.M.; Mannarino, C.F.; Resende, P.C.; Motta, F.C.; Eppinghaus, A.L.F.; Chagas Do Vale, V.H.; Braz, R.M.S.; De Andrade, J.D.S.R.; Maranhão, A.G.; et al. Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res. 2021, 191, 116810. [Google Scholar] [CrossRef]
- Rossmann, K.; Grossmann, G.; Frangoulidis, D.; Clasen, R.; Munch, M.; Hasenknopf, M.; Wurzbacher, C.; Tiehm, A.; Stange, C.; Ho, J.N.; et al. Innovative SARS-CoV-2 crisis management in the public health sector: Corona dashboard and wastewater surveillance using the example of Berchtesgadener Land, Germany. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2021, 65, 367–377. [Google Scholar] [CrossRef]
- Shah, S.; Gwee, S.X.W.; Ng, J.Q.X.; Lau, N.; Koh, J.Y.; Pang, J.X. Wastewater surveillance to infer COVID-19 transmission: A systematic review. Sci. Total Environ. 2022, 804, 150060. [Google Scholar] [CrossRef]
- Li, B.; Di, D.Y.W.; Saingam, P.; Jeon, M.K.; Yan, T. Fine-scale temporal dynamics of SARS-CoV-2 RNA abundance in wastewater during a COVID-19 lockdown. Water Res. 2021, 197, 117093. [Google Scholar] [CrossRef] [PubMed]
- Street, R.; Mathee, A.; Mangwana, N.; Dias, S.; Sharma, J.R.; Ramharack, P.; Louw, J.; Reddy, T.; Brocker, L.; Surujlal-Naicker, S.; et al. Spatial and temporal trends of SARS-CoV-2 RNA from wastewater treatment plants over 6 weeks in Cape Town, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 12085. [Google Scholar] [CrossRef]
- Brouwer, A.F.; Eisenberg, J.N.S.; Pomeroy, C.D.; Shulman, L.M.; Hindiyeh, M.; Manor, Y.; Grotto, I.; Koopman, J.S.; Eisenberg, M.C. Epidemiology of the silent polio outbreak in Rahat, Israel, based on modeling of environmental surveillance data. Proc. Natl. Acad. Sci. USA 2018, 115, E10625–E10633. [Google Scholar] [CrossRef]
- Wells, C.R.; Huppert, A.; Fitzpatrick, M.C.; Pandey, A.; Velan, B.; Singer, B.H.; Bauch, C.T.; Galvani, A.P. Prosocial polio vaccination in Israel. Proc. Natl. Acad. Sci. USA 2020, 117, 13138–13144. [Google Scholar] [CrossRef]
- Wu, F.Q.; Xiao, A.; Zhang, J.B.; Moniz, K.; Endo, N.; Armas, F.; Bushman, M.; Chai, P.R.; Duvallet, C.; Erickson, T.B.; et al. Wastewater surveillance of SARS-CoV-2 across 40 US states from February to June 2020. Water Res. 2021, 202, 117400. [Google Scholar] [CrossRef] [PubMed]
- Augusto, M.R.; Claro, I.C.M.; Siqueira, A.K.; Sousa, G.S.; Caldereiro, C.R.; Duran, A.F.A.; de Miranda, T.B.; Camillo, L.D.B.; Cabral, A.D.; Bueno, R.D. Sampling strategies for wastewater surveillance: Evaluating the variability of SARS-COV-2 RNA concentration in composite and grab samples. J. Environ. Chem. Eng. 2022, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Gerrity, D.; Papp, K.; Stoker, M.; Sims, A.; Frehner, W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: Methodology, occurrence, and incidence/prevalence considerations. Water Res. X 2021, 10, 100086. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Y.; Li, Y.; Miyani, B.; Spooner, M.; Gentry, Z.; Jacobi, S.; David, R.E.; Withington, S.; McFarlane, S.; et al. Five-week warning of COVID-19 peaks prior to the Omicron surge in Detroit, Michigan using wastewater surveillance. Sci. Total Environ. 2022, 844, 157040. [Google Scholar] [CrossRef] [PubMed]
- Layton, B.A.; Kaya, D.; Kelly, C.; Williamson, K.J.; Alegre, D.; Bachhuber, S.M.; Banwarth, P.G.; Bethel, J.W.; Carter, K.; Dalziel, B.D.; et al. Evaluation of a wastewater-based epidemiological approach to estimate the prevalence of SARS-CoV-2 infections and the detection of viral variants in disparate Oregon communities at city and neighborhood scales. Environ. Health Perspect. 2022, 130, 15. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Campagna, M.; Cricelli, L.; Grimaldi, M.; Strazzullo, S. COVID-19-related innovations: A study on underlying motivations and inter-organizational collaboration. Ind. Mark. Manag. 2022, 106, 58–70. [Google Scholar] [CrossRef]
- Harris-Lovett, S.; Nelson, K.; Beamer, P.; Bischel, H.N.; Bivins, A.; Bruder, A.; Butler, C.; Camenisch, T.D.; Long, S.K.D.; Karthikeyan, S.; et al. Wastewater surveillance for SARS-CoV-2 on college campuses: Initial efforts, lessons learned and research needs. Int. J. Environ. Res. Public Health 2021, 18, 4455. [Google Scholar] [CrossRef]
- West, N.W.; Vasquez, A.A.; Bahmani, A.; Khan, M.F.; Hartrick, J.; Turner, C.L.; Shuster, W.; Ram, J.L. Sensitive detection of SARS-CoV-2 molecular markers in urban community sewersheds using automated viral RNA purification and digital droplet PCR. Sci. Total Environ. 2022, 847, 157547. [Google Scholar] [CrossRef]
- Ram, J.L.; Thompson, B.; Turner, C.; Nechvatal, J.M.; Sheehan, H.; Bobrin, J. Identification of pets and raccoons as sources of bacterial contamination of urban storm sewers using a sequence-based bacterial source tracking method. Water Res. 2007, 41, 3605–3614. [Google Scholar] [CrossRef]
- Hillary, L.S.; Farkas, K.; Maher, K.H.; Lucaci, A.; Thorpe, J.; Distaso, M.A.; Gaze, W.H.; Paterson, S.; Burke, T.; Connor, T.R.; et al. Monitoring SARS-CoV-2 in municipal wastewater to evaluate the success of lockdown measures for controlling COVID-19 in the UK. Water Res. 2021, 200, 117214. [Google Scholar] [CrossRef]
- Amereh, F.; Negahban-Azar, M.; Isazadeh, S.; Dabiri, H.; Masihi, N.; Jahangiri-Rad, M.; Rafiee, M. Sewage systems surveillance for SARS-CoV-2: Identification of knowledge gaps, emerging threats, and future research needs. Pathogens 2021, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Gable, L.; Ram, N.; Ram, J.L. Legal and Ethical Implications of Wastewater SARS-CoV-2 Monitoring for COVID-19 Surveillance, Supplement. J. Law Biosci. 2020, 7. Available online: https://academic.oup.com/jlb/article/7/1/lsaa039/5861905#supplementary-data (accessed on 28 June 2023). [CrossRef] [PubMed]
- Ram, N.; Gable, L.; Ram, J.L. The future of wastewater monitoring for the public health. Univ. Richmond Law Rev. 2022, 56, 911–952. [Google Scholar] [CrossRef]
- Water Research Foundation. Understanding the Factors That Affect the Detection and Variability of SARS-CoV-2 in Wastewater. 2020. 6p. Available online: https://www.waterrf.org/sites/default/files/file/2020-07/RFQ_5093.pdf (accessed on 29 May 2022).
- Quinn, K.L.; Abdel-Qadir, H.; Barrett, K.; Bartsch, E.; Beaman, A.; Biering-Sørensen, T.; Colacci, M.; Cressman, A.; Detsky, A.; Gosset, A.; et al. Variation in the risk of death due to COVID-19: An international multicenter cohort study of hospitalized adults. J. Hosp. Med. 2022, 17, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Lau-Ng, R.; Caruso, L.B.; Perls, T.T. COVID-19 deaths in long-term care facilities: A critical piece of the pandemic puzzle. J. Am. Geriatr. Soc. 2020, 68, 1895–1898. [Google Scholar] [CrossRef]
- Shen, K. Relationship between nursing home COVID-19 outbreaks and staff neighborhood characteristics. PLoS ONE 2022, 17, e0267377. [Google Scholar] [CrossRef]
- Gable, L.; Ram, N.; Ram, J.L. Legal and ethical implications of wastewater SARS-CoV-2 monitoring for COVID-19 surveillance. J. Law Biosci. 2020, 7, lsaa039. [Google Scholar] [CrossRef]
- Michigan.gov. Coronavirus/Michigan Data. Long Term Care Data. 2022. Available online: https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173-526911--,00.html (accessed on 10 April 2022).
- Zhou, Y.; Zhi, H.; Teng, Y. The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity. J. Med. Virol. 2022, 95, e28138. [Google Scholar] [CrossRef]
- Oloye, F.F.; Xie, Y.W.; Asadi, M.; Cantin, J.; Challis, J.K.; Brinkmann, M.; McPhedran, K.N.; Kristian, K.; Keller, M.; Sadowski, M.; et al. Rapid transition between SARS-CoV-2 variants of concern Delta and Omicron detected by monitoring municipal wastewater from three Canadian cities. Sci. Total Environ. 2022, 841, 156741. [Google Scholar] [CrossRef]
- Wilhelm, A.; Agrawal, S.; Schoth, J.; Meinert-Berning, C.; Bastian, D.; Orschler, L.; Ciesek, S.; Teichgräber, B.; Wintgens, T.; Lackner, S.; et al. Early detection of SARS-CoV-2 Omicron BA.4 and BA.5 in German wastewater. Viruses 2022, 14, 1876. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Schmitz, B.W.; Innes, G.K.; Prasek, S.M.; Pogreba Brown, K.M.; Stark, E.R.; Foster, A.R.; Sprissler, R.S.; Harris, D.T.; Sherchan, S.P.; et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021, 779, 146408. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, W.W.; Schmitz, B.W.; Innes, G.K.; Pogreba Brown, K.M.; Prasek, S.M.; Stark, E.R.; Foster, A.R.; Sprissler, R.S.; Harris, D.T.; Sherchan, S.P.; et al. Wastewater-based epidemiology for averting COVID-19 outbreaks on the University of Arizona campus. medRxiv 2020. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC) US Dept. of Public Health Services. 2019-Novel Coronavirus (2019-nCoV) Real-Time rRT-PCR Panel Primers and Probes, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases. 2020; 2p. Available online: https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf (accessed on 28 June 2023).
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, J.; Xiao, A.; Gu, X.; Lee, W.L.; Armas, F.; Kauffman, K.; Hanage, W.; Matus, M.; Ghaeli, N.; et al. SARS-CoV-2 Titers in Wastewater Are Higher than Expected from Clinically Confirmed Cases. mSystems 2020, 5, e00614-20. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Musso, N.; Gattuso, G.; Bongiorno, D.; Palermo, C.I.; Scalia, G.; Libra, M.; Stefani, S. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int. J. Mol. Med. 2020, 46, 957–964. [Google Scholar] [CrossRef]
- Flood, M.T.; D’Souza, N.; Rose, J.B.; Aw, T.G. Methods evaluation for rapid concentration and quantification of SARS-CoV-2 in raw wastewater using droplet digital and quantitative RT-PCR. Food Environ. Virol. 2021, 13, 303–315. [Google Scholar] [CrossRef]
- Smith, K. Is Michigan Prepared for the Next COVID-19 Surge? Wastewater Testing May Help. WXYZ 2022. Available online: https://www.wxyz.com/news/coronavirus/is-michigan-prepared-for-the-next-covid-19-surge-wastewater-testing-may-help (accessed on 25 October 2022).
- McClary-Gutierrez, J.S.; Mattioli, M.C.; Marcenac, P.; Silverman, A.I.; Boehm, A.B.; Bibby, K.; Balliet, M.; de los Reyes, F.L.; Gerrity, D.; Griffith, J.F.; et al. SARS-CoV-2 Wastewater Surveillance for Public Health Action. Emerg. Infect. Dis. 2021, 27, 1–8. [Google Scholar] [CrossRef]
- Geurts, A.; Geerdink, T.; Sprenkeling, M. Accelerated innovation in crises: The role of collaboration in the development of alternative ventilators during the COVID-19 pandemic. Technol. Soc. 2022, 68, 12. [Google Scholar] [CrossRef]
- Hrudey, S.E.; Bischel, H.N.; Charrois, J.; Chik, A.H.S.; Conant, B.; Delatolla, R.; Dorner, S.; Graber, T.E.; Hubert, C.; Isaac-Renton, J.; et al. Wastewater surveillance for SARS-CoV-2 RNA in Canada. Facets 2022, 7, 1493–1597. [Google Scholar] [CrossRef]
- Sims, N.; Kasprzyk-Hordern, B. Future perspectives of wastewater-based epidemiology: Monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020, 139, 105689. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVID Data Tracker. 2023. Available online: https://covid.cdc.gov/covid-data-tracker/#wastewater-surveillance (accessed on 22 June 2023).
- Wartell, B.A.; Proano, C.; Bakalian, L.; Kaya, D.; Croft, K.; McCreary, M.; Lichtenstein, N.; Miske, V.; Arcellana, P.; Boyer, J.; et al. Implementing wastewater surveillance for SARS-CoV-2 on a university campus: Lessons learned. Water Environ. Res. 2022, 94, 10807. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Kumar, S.; Rathore, P.; Sarma, R.; Malhotra, R.K.; Choudhary, N.; Thankachan, A.; Haokip, N.; Singh, S.; Pandit, A.; et al. Surviving COVID-19 is half the battle; living life with perceived stigma is other half: A cross-sectional study. Indian J. Psychol. Med. 2021, 43, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Smith-Morris, C. Epidemiological placism in public health emergencies: Ebola in two Dallas neighborhoods. Soc. Sci. Med. 2017, 179, 106–114. [Google Scholar] [CrossRef]
- Price, M.; Trowsdale, S. The ethics of wastewater surveillance for public health. J. Hydrol. N. Z. 2022, 61, 59–75. [Google Scholar]
- Spurbeck, R.R.; Minard-Smith, A.; Catlin, L. Feasibility of neighborhood and building scale wastewater-based genomic epidemiology for pathogen surveillance. Sci. Total Environ. 2021, 789, 8. [Google Scholar] [CrossRef]
- Shrestha, S.; Yoshinaga, E.; Chapagain, S.K.; Mohan, G.; Gasparatos, A.; Fukushi, K. Wastewater-based epidemiology for cost-effective mass surveillance of COVID-19 in low- and middle-income countries: Challenges and opportunities. Water 2021, 13, 2897. [Google Scholar] [CrossRef]
- Naughton, C.C.; Holm, R.; Lin, N.J.J.; James, B.P.; Smith, T. Online dashboards for SARS-CoV-2 wastewater data need standard best practices: An environmental health communication agenda. J. Water Health 2023, 21, 615–624. [Google Scholar] [CrossRef]
- Ram, N.; Shuster, W.; Gable, L.; Ram, J.L. Ethical and legal wastewater surveillance. Science 2023, 379, 652. [Google Scholar] [CrossRef]
- Khoury, M.J.; Armstrong, G.L.; Bunnell, R.E.; Cyril, J.; Iademarco, M.F. The intersection of genomics and big data with public health: Opportunities for precision public health. PLoS Med 2020, 17, 14. [Google Scholar] [CrossRef]
- Khoury, M.J.; Bowen, S.; Dotson, W.D.; Drzymalla, E.; Green, R.F.; Goldstein, R.; Kolor, K.; Liburd, L.C.; Sperling, L.S.; Bunnell, R. Health equity in the implementation of genomics and precision medicine: A public health imperative. Genet. Med. 2022, 24, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.A.; West, N.W.; Bahmani, A.; Ram, J.L. Rapid and Direct Method to Extract SARS-CoV-2 RNA from Municipal Wastewater Using the CHEMAGIC 360™ 12-Rod Head Platform. 2021. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ram, J.L.; Shuster, W.; Gable, L.; Turner, C.L.; Hartrick, J.; Vasquez, A.A.; West, N.W.; Bahmani, A.; David, R.E. Wastewater Monitoring for Infectious Disease: Intentional Relationships between Academia, the Private Sector, and Local Health Departments for Public Health Preparedness. Int. J. Environ. Res. Public Health 2023, 20, 6651. https://doi.org/10.3390/ijerph20176651
Ram JL, Shuster W, Gable L, Turner CL, Hartrick J, Vasquez AA, West NW, Bahmani A, David RE. Wastewater Monitoring for Infectious Disease: Intentional Relationships between Academia, the Private Sector, and Local Health Departments for Public Health Preparedness. International Journal of Environmental Research and Public Health. 2023; 20(17):6651. https://doi.org/10.3390/ijerph20176651
Chicago/Turabian StyleRam, Jeffrey L., William Shuster, Lance Gable, Carrie L. Turner, James Hartrick, Adrian A. Vasquez, Nicholas W. West, Azadeh Bahmani, and Randy E. David. 2023. "Wastewater Monitoring for Infectious Disease: Intentional Relationships between Academia, the Private Sector, and Local Health Departments for Public Health Preparedness" International Journal of Environmental Research and Public Health 20, no. 17: 6651. https://doi.org/10.3390/ijerph20176651
APA StyleRam, J. L., Shuster, W., Gable, L., Turner, C. L., Hartrick, J., Vasquez, A. A., West, N. W., Bahmani, A., & David, R. E. (2023). Wastewater Monitoring for Infectious Disease: Intentional Relationships between Academia, the Private Sector, and Local Health Departments for Public Health Preparedness. International Journal of Environmental Research and Public Health, 20(17), 6651. https://doi.org/10.3390/ijerph20176651