Mixing Meds and Milk: Evaluation of a Performance Gap Intervention for Provider Education in Breastfeeding and Maternal Medication Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Program Evaluation Design
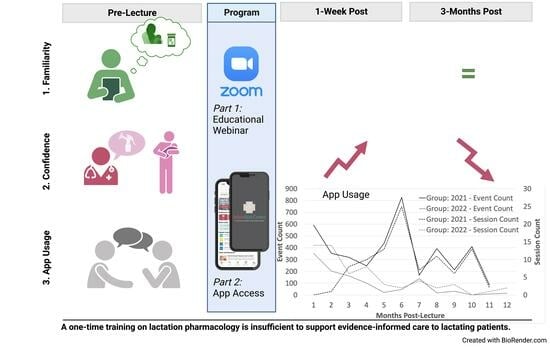
2.2. Educational Intervention
2.3. Survey Data
2.4. App Data
3. Results
3.1. Survey Data
3.1.1. Sample Features
3.1.2. Sample Familiarity and Confidence
3.1.3. Responses to Training
3.2. InfantRisk HCP App Data
4. Discussion
4.1. Survey Data
4.2. App Data
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sattari, M.; Serwint, J.R.; Levine, D.M. Maternal Implications of Breastfeeding: A Review for the Internist. Am. J. Med. 2019, 132, 912–920. [Google Scholar] [CrossRef]
- Qiu, R.; Zhong, Y.; Hu, M.; Wu, B. Breastfeeding and Reduced Risk of Breast Cancer: A Systematic Review and Meta-Analysis. Comput. Math. Methods Med. 2022, 2022, 8500910. [Google Scholar] [CrossRef]
- Bartick, M.C.; Schwarz, E.B.; Green, B.D.; Jegier, B.J.; Reinhold, A.G.; Colaizy, T.T.; Bogen, D.L.; Schaefer, A.J.; Stuebe, A.M. Suboptimal breastfeeding in the United States: Maternal and pediatric health outcomes and costs. Matern. Child Nutr. 2017, 13, e12366. [Google Scholar] [CrossRef]
- 2020 Breastfeeding Report Card; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Saucedo Baza, A.; Mignacca, C.; Delgado, P.E.; Paterniti, T.A.; Romero de Mello Sa, S.; Looney, S.; Zahler-Miller, C. A Technological Approach to Improved Breastfeeding Rates and Self-Efficacy: A Randomized Controlled Pilot Study. J. Hum. Lact. 2023. [Google Scholar] [CrossRef] [PubMed]
- Blixt, I.; Rosenblad, A.K.; Axelsson, O.; Funkquist, E.L. Breastfeeding training improved healthcare professional’s self-efficacy to provide evidence-based breastfeeding support: A pre-post intervention study. Midwifery 2023, 125, 103794. [Google Scholar] [CrossRef] [PubMed]
- Blixt, I.; Martensson, L.B.; Ekstrom, A.C. Process-oriented training in breastfeeding for health professionals decreases women’s experiences of breastfeeding challenges. Int. Breastfeed. J. 2014, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Sachs, H.C.; Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics 2013, 132, e796–e809. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.M., Jr.; Paul, I.M.; Vesell, E.S. Safety issues of maternal drug therapy during breastfeeding. Clin. Pharmacol. Ther. 2009, 85, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Davanzo, R.; Bua, J.; De Cunto, A.; Farina, M.L.; De Ponti, F.; Clavenna, A.; Mandrella, S.; Sagone, A.; Clementi, M. Advising Mothers on the Use of Medications during Breastfeeding: A Need for a Positive Attitude. J. Hum. Lact. 2016, 32, 15–19. [Google Scholar] [CrossRef]
- Scime, N.V.; Patten, S.B.; Tough, S.C.; Chaput, K.H. Maternal chronic disease and breastfeeding outcomes: A Canadian population-based study. J. Matern.-Fetal Neonatal Med. 2022, 35, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Blixt, I.; Johansson, M.; Hildingsson, I.; Papoutsi, Z.; Rubertsson, C. Women’s advice to healthcare professionals regarding breastfeeding: “Offer sensitive individualized breastfeeding support”—An interview study. Int. Breastfeed. J. 2019, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Bowes, W.A., Jr. The effect of medications on the lactating mother and her infant. Clin. Obstet. Gynecol. 1980, 23, 1073–1080. [Google Scholar] [CrossRef]
- Kirkland, V.L. Maternal Report of Reasons Related to Breastfeeding Cessation during the First Year Postpartum: A Descriptive Nationally Based Study. Ph.D. Thesis, Rutgers The State University of New Jersey-Newark, Ann Arbor, NJ, USA, 2001. [Google Scholar]
- Louis-Jacques, A.F.; Wilson, R.; Dean, K.; Hernandez, I.; Spatz, D.; Obican, S. Improving Drug Exposure Knowledge during Lactation: Quality Improvement Initiative in Low-Income Women. Breastfeed. Med. 2020, 15, 140–146. [Google Scholar] [CrossRef]
- Liang, O.S.; Sheffield, J.S.; Taylor, C.O. Detecting Patterns of Prescription Drug Use During Pregnancy and Lactation with Visualization Techniques. AMIA Jt. Summits Transl. Sci. Proc. 2019, 2019, 478–487. [Google Scholar]
- Ramdas, D.; Drury, N.; Jordan, C.; Panda, S.; Singh, A.P. Effects of Infant Driven Feeding Program on Provision of Breast Milk in Very Low Birth Weight Infants. Breastfeed. Med. 2023, 18, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Bloom, B.S.; Krathwohl, D.R. Taxonomy of Educational Objectives: The Classification of Educational Goals; Longmans, Green & Co.: London, UK, 1956. [Google Scholar]



| Item | Pre-Survey N (%) | Post 1 N (%) | Post 2 N (%) | p-Value a (Pre-Post 1) | p-Value a (Pre-Post 2) |
|---|---|---|---|---|---|
| Familiarity for medication safety in those used for… | |||||
| Common conditions | n/a b | 0.797 (n = 11) | |||
| Not at all familiar (1) | 2 (4.2) | n/a | 1 (2.1) | ||
| Slightly familiar (2) | 11 (22.9) | n/a | 6 (12.5) | ||
| Very familiar (3) | 19 (39.6) | n/a | 7 (14.6) | ||
| Extremely familiar (4) | 7 (14.6) | n/a | 4 (8.3) | ||
| Missing | 9 (18.8) | n/a | 30 (62.5) | ||
| Chronic conditions | n/a | 1.000 (n = 18) | |||
| Not at all familiar (1) | 3 (6.3) | n/a | 0 (0.0) | ||
| Slightly familiar (2) | 16 (33.3) | n/a | 7 (14.6) | ||
| Very familiar (3) | 21 (43.8) | n/a | 9 (18.8) | ||
| Extremely familiar (4) | 6 (4.2) | n/a | 2 (4.2) | ||
| Missing | 2 (4.2) | n/a | 30 (62.5) | ||
| Unique conditions | n/a | 0.3008 (n = 18) | |||
| Not at all familiar (1) | 29 (60.4) | n/a | 10 (20.8) | ||
| Slightly familiar (2) | 15 (31.3) | n/a | 5 (10.4) | ||
| Very familiar (3) | 1 (2.1) | n/a | 2 (4.2) | ||
| Extremely familiar (4) | 1 (2.1) | n/a | 1 (2.1) | ||
| Missing | 2 (4.2) | n/a | 30 (62.5) | ||
| Multiple simultaneous medications | n/a | 0.680 (n = 18) | |||
| Not at all familiar (1) | 15 (31.3) | n/a | 6 (12.5) | ||
| Slightly familiar (2) | 27 (56.3) | n/a | 9 (18.8) | ||
| Very familiar (3) | 3 (6.3) | n/a | 2 (4.2) | ||
| Extremely familiar (4) | 1 (2.1) | n/a | 1 (2.1) | ||
| Missing | 2 (4.2) | n/a | 30 (62.5) | ||
| Confidence to make recommendations in regard to medicine for… | |||||
| Common conditions | 0.000 (n = 39) | 0.531 (n = 17) | |||
| Not at all confident (1) | 4 (8.3) | 0 (0.0) | 3 (6.3) | ||
| Slightly confident (2) | 22 (45.8) | 5 (10.4) | 7 (14.6) | ||
| Very confident (3) | 16 (33.3) | 27 (56.3) | 6 (12.5) | ||
| Extremely confident (4) | 3 (6.3) | 9 (18.8) | 1 (2.1) | ||
| Missing | 3 (6.5) | 7 (14.6) | 31 (64.6) | ||
| Chronic conditions | 0.000 (n = 36) | 1.000 (n = 16) | |||
| Not at all confident (1) | 6 (12.5) | 1 (2.1) | 2 (4.2) | ||
| Slightly confident (2) | 25 (52.1) | 7 (14.6) | 8 (16.7) | ||
| Very confident (3) | 10 (20.8) | 27 (56.3) | 6 (12.5) | ||
| Extremely confident (4) | 3 (6.3) | 4 (8.3) | 1 (2.1) | ||
| Missing | 4 (8.3) | 9 (18.7) | 31 (64.6) | ||
| Unique conditions | 0.000 (n = 34) | 0.656 (n = 16) | |||
| Not at all confident (1) | 26 (54.2) | 4 (8.3) | 10 (20.8) | ||
| Slightly confident (2) | 13 (27.1) | 19 (39.6) | 5 (10.4) | ||
| Very confident (3) | 4 (8.3) | 12 (25.0) | 1 (2.1) | ||
| Extremely confident (4) | 0 (0.0) | 3 (6.3) | 1 (2.1) | ||
| Missing | 5 (10.4) | 10 (20.8) | 31 (64.6) | ||
| Multiple simultaneous medications | 0.000 (n = 35) | 0.688 (n = 16) | |||
| Not at all confident (1) | 21 (43.8) | 5 (10.4) | 8 (16.7) | ||
| Slightly confident (2) | 19 (39.6) | 13 (27.1) | 6 (12.5) | ||
| Very confident (3) | 4 (8.3) | 18 (37.5) | 2 (4.2) | ||
| Extremely confident (4) | 0 (0.0) | 2 (4.2) | 1 (2.1) | ||
| Missing | 4 (8.3) | 10 (20.8) | 31 (64.6) | ||
| When making recommendations, how often did you use the following resources? | |||||
| Personal experience | n/a | 0.410 (n = 17) | |||
| Never (1) | 16 (33.4) | n/a | 8 (16.7) | ||
| Seldom (2) | 10 (20.8) | n/a | 3 (6.3) | ||
| Often (3) | 15 (31.3) | n/a | 4 (8.3) | ||
| Always (4) | 4 (8.3) | n/a | 3 (6.3) | ||
| Missing | 3 (6.2) | n/a | 30 (62.5) | ||
| A trusted colleague | n/a | 0.125 (n = 17) | |||
| Never (1) | 9 (18.8) | n/a | 4 (8.3) | ||
| Seldom (2) | 11 (22.9) | n/a | 6 (12.5) | ||
| Often (3) | 19 (39.6) | n/a | 6 (12.5) | ||
| Always (4) | 6 (12.5) | n/a | 2 (4.2) | ||
| Missing | 3 (6.2) | n/a | 30 (62.5) | ||
| Internet search | n/a | 0.699 (n = 17) | |||
| Never (1) | 12 (25.0) | n/a | 6 (12.5) | ||
| Seldom (2) | 17 (35.4) | n/a | 3 (6.3) | ||
| Often (3) | 14 (29.2) | n/a | 8 (16.7) | ||
| Always (4) | 2 (4.2) | n/a | 1 (2.1) | ||
| Missing | 3 (6.2) | n/a | 30 (62.5) | ||
| Literature search | n/a | 0.844 (n = 17) | |||
| Never (1) | 7 (14.6) | n/a | 5 (10.4) | ||
| Seldom (2) | 10 (20.8) | n/a | 3 (6.3) | ||
| Often (3) | 20 (41.7) | n/a | 6 (12.5) | ||
| Always (4) | 8 (16.7) | n/a | 4 (8.3) | ||
| Missing | 3 (6.2) | n/a | 30 (62.5) | ||
| Information from drug manufacturer | n/a | 0.281 (n = 18) | |||
| Never (1) | 15 (31.3) | n/a | 5 (10.4) | ||
| Seldom (2) | 15 (31.3) | n/a | 8 (16.7) | ||
| Often (3) | 10 (20.8) | n/a | 3 (6.3) | ||
| Always (4) | 6 (12.5) | n/a | 2 (4.2) | ||
| Missing | 2 (4.1) | n/a | 30 (62.5) | ||
| Medical references | n/a | 0.699 (n = 18) | |||
| Never (1) | 15 (31.3) | n/a | 6 (12.5) | ||
| Seldom (2) | 7 (14.6) | n/a | 7 (14.6) | ||
| Often (3) | 17 (35.3) | n/a | 3 (6.3) | ||
| Always (4) | 7 (14.6) | n/a | 2 (4.2) | ||
| Missing | 2 (4.2) | n/a | 30 (62.5) | ||
| Lactation-specific reference | n/a | 1.000 (n = 18) | |||
| Never (1) | 4 (8.3) | n/a | 1 (2.1) | ||
| Seldom (2) | 1 (2.1) | n/a | 2 (4.2) | ||
| Often (3) | 13 (27.1) | n/a | 4 (8.3) | ||
| Always (4) | 28 (58.3) | n/a | 11 (22.9) | ||
| Missing | 2 (4.2) | n/a | 30 (62.5) | ||
| Pre-Survey Characteristic | Baseline Sample n (%) | Analytic Sample n (%) |
|---|---|---|
| Role | ||
| Nurse/Lactation Professional | 63 (61.2%) | 33 (68.8%) |
| Nurse Practitioner/Midwife | 9 (8.7%) | 3 (6.3%) |
| Physician | 13 (12.6%) | 3 (6.3%) |
| Other/Undisclosed | 18 (17.5%) | 4 (8.3%) |
| How frequently do you encounter the issue of medication use in breastfeeding women? | ||
| Daily | 18 (17.5%) | 8 (16.7%) |
| Several times a week | 23 (22.3%) | 13 (27.1%) |
| Once a week | 17 (16.5%) | 8 (16.7%) |
| 2–3 times a month | 15 (14.6%) | 9 (18.8%) |
| Monthly | 9 (8.7%) | 4 (8.3%) |
| Less often | 15 (14.6%) | 5 (10.4%) |
| Missing | 6 (5.8%) | 1 (2.1%) |
| To what extent do you have personal experience with breastfeeding, either for yourself, a family member, or a friend? | ||
| A large amount (4) | 54 (52.4%) | 26 (54.2%) |
| A moderate amount | 35 (34.0%) | 17 (35.4%) |
| A small amount | 12 (11.7%) | 4 (8.3%) |
| No experience (1) | 2 (1.9%) | 1 (2.1%) |
| Missing | 0 (0%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohn, K.; Fernandez, A.; Stroever, S.; O’Neil, D.; Enderle, J.; Krutsch, K. Mixing Meds and Milk: Evaluation of a Performance Gap Intervention for Provider Education in Breastfeeding and Maternal Medication Use. Int. J. Environ. Res. Public Health 2023, 20, 6850. https://doi.org/10.3390/ijerph20196850
Bohn K, Fernandez A, Stroever S, O’Neil D, Enderle J, Krutsch K. Mixing Meds and Milk: Evaluation of a Performance Gap Intervention for Provider Education in Breastfeeding and Maternal Medication Use. International Journal of Environmental Research and Public Health. 2023; 20(19):6850. https://doi.org/10.3390/ijerph20196850
Chicago/Turabian StyleBohn, Kaci, Alejandra Fernandez, Stephanie Stroever, Dara O’Neil, Joan Enderle, and Kaytlin Krutsch. 2023. "Mixing Meds and Milk: Evaluation of a Performance Gap Intervention for Provider Education in Breastfeeding and Maternal Medication Use" International Journal of Environmental Research and Public Health 20, no. 19: 6850. https://doi.org/10.3390/ijerph20196850
APA StyleBohn, K., Fernandez, A., Stroever, S., O’Neil, D., Enderle, J., & Krutsch, K. (2023). Mixing Meds and Milk: Evaluation of a Performance Gap Intervention for Provider Education in Breastfeeding and Maternal Medication Use. International Journal of Environmental Research and Public Health, 20(19), 6850. https://doi.org/10.3390/ijerph20196850