Incidence and Distribution of Microfungi in a Treated Municipal Water Supply System in Sub-Tropical Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Supply System
2.2. Sample Collection
2.3. Physico-chemical Parameters
2.4. Microfungal Analyses
2.5. Bacteriological Analyses
2.6. Statistical Analyses
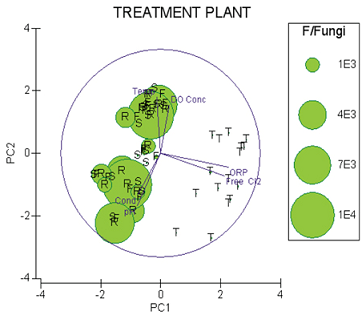
3. Results
3.1. Microfungal Enumeration and Identification
3.2. Statistical Analyses
4. Discussion
Acknowledgments
References
- LeChevallier, MW; Norton, WD; Lee, RG. Giardia and Cryptosporidium spp. in filtered drinking water supplies. Appl. Environ. Microbiol 1991, 57, 2617–2621. [Google Scholar]
- LeChevallier, MW; Norton, WD; Lee, RG. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl. Environ. Microbiol 1991, 57, 2610–2616. [Google Scholar]
- Mac Kenzie, WR; Hoxie, NJ; Proctor, ME; Gradus, MS; Blair, KA; Peterson, DE; Kazmierczak, JJ; Addiss, DG; Fox, KR; Rose, JB; Davis, JP. A massive outbreak in milwaukee of cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med 1994, 331, 161–167. [Google Scholar]
- Wallis, PM; Erlandsen, SL; Isaac-Renton, JL; Olson, ME; Robertson, WJ; van Keulen, H. Prevalence of Giardia cysts and Cryptosporidium oocysts and characterization of Giardia spp. isolated from drinking water in Canada. Appl. Environ. Microbiol 1996, 62, 2789–2797. [Google Scholar]
- Alvarez, CIV. Caracterización micológica de aquas “crudas” y filtradas en la Planta de Tratamiento de Tres Ríos, Costa Rica. Rev. Biol. Trop 1993, 41, 417–422. [Google Scholar]
- Goncalves, AB; Russell, R; Paterson, M; Lima, N. Survey and significance of filamentous fungi from tap water. Int. J. Hyg. Environ. Health 2005, 209, 257–264. [Google Scholar]
- Göttlich, E; van der Lubbe, W; Lange, B; Fiedler, S; Melchert, I; Reifenrath, M; Flemming, H-C; de Hoog, S. Fungal flora in groundwater-derived public drinking water. Int. J. Hyg. Environ. Health 2002, 200, 269–279. [Google Scholar]
- Hageskal, G; Gaustad, P; Heier, BT; Skaar, I. Occurrence of moulds in drinking water. J. Appl. Microbiol 2007, 102, 774–780. [Google Scholar]
- Hageskal, G; Knutsen, AK; Gaustad, P; de Hoog, GS; Skaar, I. Diversity and significance of mold species in Norwegian drinking water. Appl. Environ. Microbiol 2006, 72, 7586–7593. [Google Scholar]
- Hinzelin, F; Block, JC. Yeasts and filamentous fungi in drinking water. Environ. Technol. Lett 1985, 6, 101–106. [Google Scholar]
- Kanzler, D; Buzina, W; Paulitsch, A; Haas, D; Platzer, S; Marth, E; Mascher, F. Occurrence and hygienic relevance of fungi in drinking water. Mycoses (OnlineEarly Articles) 2007, 51, 165–169. [Google Scholar]
- Kelley, J; Kinsey, G; Paterson, R; Brayford, D; Pitchers, R; Rossmoore, H. Identification and Control of Fungi in Distribution Systems; AWWA Research Foundation and American Water Works Association: Denver, CO, USA, 2003. [Google Scholar]
- Nagy, LA; Olson, BH. The occurrence of filamentous fungi in drinking water distribution systems. Can. J. Microbiol 1982, 28, 667–671. [Google Scholar]
- Niemi, RM; Knuth, S; Lundstrom, K. Actinomycetes and fungi in surface waters and in potable water. Appl. Environ. Microbiol 1982, 43, 378–388. [Google Scholar]
- Rosenzweig, WD; Minnigh, H; Pipes, WO. Fungi in potable water distribution systems. J. Am. Water Works Assoc 1986, 78, 53–55. [Google Scholar]
- West, PR. Isolation rates and characterisation of fungi in drinking water systems. In Proceedings of the Water Quality Technology Conference; AWWA Research Foundation and the American Water Works Association: Denver, CO, USA, 1986; pp. 457–473. [Google Scholar]
- Yamaguchi, MU; Rampazzo, RCP; Yamada-Ogatta, SF; Nakamura, CV; Ueda-Nakamura, T; Filho, BPD. Yeasts and filamentous fungi in bottled mineral water and tap water from municipal supplies. Braz. Arch. Biol. Technol 2007, 50, 1–9. [Google Scholar]
- Anaissie, EJ; Costa, SF. Nosocomial aspergillosis is waterborne. Clin. Infect. Dis 2001, 33, 1546–1548. [Google Scholar]
- Anaissie, EJ; Stratton, SL; Dignani, MC; Summerbell, RC; Rex, JH; Monson, TP; Spencer, T; Kasai, M; Francesconi, A; Walsh, TJ. Pathogenic Aspergillus species recovered from a hospital water system: a 3-year prospective study. Clin. Infect. Dis 2002, 34, 780–789. [Google Scholar]
- Arvanitidou, M; Kanellou, K; Constantinides, TC; Katsouyannopoulos, V. The occurrence of fungi in hospital and community potable waters. Lett. Appl. Microbiol 1999, 29, 81–84. [Google Scholar]
- Hapcioglu, B; Yegenoglu, Y; Erturan, Z; Nakipoglu, Y; Issever, H. Heterotrophic bacteria and filamentous fungi isolated from a hospital water distribution system. Indoor Environ 2005, 14, 487–493. [Google Scholar]
- Warris, A; Gaustad, P; Meis, JFGM; Voss, A; Verweij, PE. Recovery of filamentous fungi from water in a paediatric bone marrow transplantation unit. J. Hosp. Infect 2001, 47, 143–148. [Google Scholar]
- Warris, A; Voss, A; Verweij, PE. Hospital sources of Aspergillus species: New routes of transmission? Rev. Iber. Micol 2001, 18, 156–162. [Google Scholar]
- Bays, L; Burman, NP; Lewis, WM. Taste and odour in water supplies in Great Britain: a survey of the present position and problems for the future. Water Treat. Exam 1970, 19, 136–160. [Google Scholar]
- Eaton, AD; Clesceri, LS; Rice, EW; Greenberg, AE. Detection of Fungi—Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2005; Volume 21. [Google Scholar]
- Carmichael, JW; Kendrick, WB; Connors, IL; Sigler, L. Genera of Hyphomycetes; The University of Alberta Press: Edmonton, Canada, 1980. [Google Scholar]
- Ellis, MB. Dematiaceous Hyphomycetes; The Commonwealth Mycological Institute: Kew, UK, 1971. [Google Scholar]
- Ellis, MB. More Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Kew, UK, 1976. [Google Scholar]
- Kendrick, WB; Carmichael, JW. Hyphomycetes. In The Fungi; Ainsworth, GC, Sparrow, AS, Sussman, AS, Eds.; Academic Press: New York, NY, USA, 1973; Volume IV A, pp. 323–509. [Google Scholar]
- Clarke, KR; Gorley, RN. Primer v6: User Manual/Tutorial; Primer-E Ltd.: Plymouth, UK, 2006. [Google Scholar]
- Sammon, NB; Harrower, KM; Fabbro, LD; Reed, RH. Green tree frogs: Contamination of covered reservoirs in Northern Australia. Water 2009, 36, 97–101. [Google Scholar]
- Grabinska-Loniewska, A; Konillowicz-Kowalska, T; Wardzynska, G; Boryn, K. Occurrence of Fungi in Water Distribution System. Polish J. Environ. Stud 2007, 4, 539–547. [Google Scholar]
- Hageskal, G; Lima, N; Skaar, I. The study of fungi in drinking water. Mycol. Res 2009, 113, 165–172. [Google Scholar] [Green Version]
- Ramirez-toro, GI; Minnigh, HA. Attachment and colonization of fungi in potable water distribution systems. Proceedings of XXVIII Congreso Interamericano de Ingenieria Sanitaria y Ambiental, Cancon, Mexico, 27–31 October, 2002.
- Rippon, JW. Medical Mycology—The Pathogenic Fungi and the Pathogenic Actinomycetes, 3rd ed; W.B. Saunders Company: Philadelphia, PA, USA, 1988. [Google Scholar]



| Mains | Reservoirs | Treated water ex Treatment Plant | ||||
|---|---|---|---|---|---|---|
| CFU | % | CFU | % | CFU | % | |
| Genus | ||||||
| Cladosporium | 333 | 37.8 | 967 | 44.4 | 13 | 37.1 |
| Penicillium | 81 | 9.2 | 315 | 14.5 | 0 | 0.0 |
| Aspergillus | 36 | 4.1 | 119 | 5.5 | 2 | 5.7 |
| Trichoderma | 11 | 1.2 | 18 | 0.8 | 0 | 0.0 |
| Fusarium | 27 | 3.1 | 43 | 2.0 | 5 | 14.3 |
| Pithomyces | 28 | 3.2 | 39 | 1.8 | 0 | 0.0 |
| Alternaria | 22 | 2.5 | 34 | 1.6 | 0 | 0.0 |
| Paecilomyces | 29 | 3.3 | 31 | 1.4 | 0 | 0.0 |
| Acremonium | 44 | 5.0 | 8 | 0.4 | 0 | 0.0 |
| Epicoccum | 8 | 0.9 | 26 | 1.2 | 0 | 0.0 |
| Curvularia | 6 | 0.7 | 30 | 1.4 | 0 | 0.0 |
| Other genera < 1% | 120 | 13.6 | 103 | 4.7 | 3 | 8.6 |
| Asporogenous colonies | 136 | 15.4 | 443 | 20.4 | 12 | 34.3 |
| Total filamentous fungi | 881 | 100 | 2176 | 100 | 35 | 100 |
| Yeasts/yeast-like fungi | 151 | 2,699 | ||||
| Total microfungi | 1,032 | 4,875 | ||||
| MDS Biological | MDS Environmental | BEST analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| Stress | Stress | No. of var. | Corr. | Selection (variables) | ||||
| Min. | Measured | Min. | Measured | |||||
| MAINS | ||||||||
| 0.01 | 3-d | 0.00 | 0.01 | 3-d | 0.11 | 1 | 0.476 | 6 |
| 2-d | 0.01 | 2-d | 0.20 | 2 | 0.428 | 5, 6 | ||
| 3 | 0.426 | 1, 5, 6 | ||||||
| 2 | 0.399 | 1, 6 | ||||||
| Significance level = 0.001 999 permutations | ||||||||
| RESERVOIRS | ||||||||
| 0.01 | 3-d | 0.00 | 0.01 | 3-d | 0.10 | 1 | 0.291 | 6 |
| 2-d | 0.01 | 2-d | 0.20 | 2 | 0.265 | 5, 6 | ||
| 3 | 0.235 | 2, 5, 6 | ||||||
| 4 | 0.229 | 1, 2, 5, 6 | ||||||
| Significance level = 0.001 999 permutations | ||||||||
| TREATMENT PLANT | ||||||||
| 0.01 | 3-d | 0.04 | 0.01 | 3-d | 0.08 | 1 | 0.725 | 6 |
| 2-d | 0.06 | 2-d | 0.17 | 2 | 0.650 | 5, 6 | ||
| 1 | 0.636 | 5 | ||||||
| 3 | 0.634 | 3, 5, 6 | ||||||
| Significance level = 0.001 999 permutations | ||||||||
| Principal components analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC No | Eigen values | % var | Cum % var | Variables | Eigenvectors | |||||
| PC1 | PC2 | PC3 | PC4 | PC5 | ||||||
| MAINS | ||||||||||
| 1 | 2.06 | 34.3 | 34.3 | 1 | Temp | −0.254 | −0.546 | −0.129 | 0.668 | −0.418 |
| 2 | 1.33 | 22.2 | 56.5 | 2 | Cond | 0.088 | 0.484 | 0.485 | 0.667 | 0.234 |
| 3 | 1.14 | 19.0 | 75.5 | 3 | DO conc | −0.337 | −0.546 | 0.410 | −0.105 | 0.629 |
| 4 | 0.847 | 14.1 | 89.6 | 4 | pH | 0.419 | −0.129 | −0.623 | 0.237 | 0.495 |
| 5 | 0.416 | 6.9 | 96.6 | 5 | ORP | −0.598 | 0.280 | −0.191 | −0.113 | −0.102 |
| 6 | Free Cl2 | −0.530 | 0.273 | −0.395 | 0.172 | 0.346 | ||||
| RESERVOIRS | ||||||||||
| 1 | 2.24 | 37.3 | 37.3 | 1 | Temp | −0.349 | 0.244 | −0.254 | −0.768 | 0.401 |
| 2 | 1.49 | 24.8 | 62.1 | 2 | Cond | 0.141 | −0.266 | 0.805 | −0.486 | −0.146 |
| 3 | 0.972 | 16.2 | 78.4 | 3 | DO conc | −0.450 | 0.523 | 0.102 | −0.020 | −0.704 |
| 4 | 0.905 | 15.1 | 93.4 | 4 | pH | 0.403 | −0.327 | −0.521 | −0.388 | −0.542 |
| 5 | 0.256 | 4.3 | 97.7 | 5 | ORP | −0.481 | −0.514 | −0.042 | 0.149 | 0.043 |
| 6 | Free Cl2 | −0.512 | −0.474 | −0.064 | −0.038 | −0.162 | ||||
| TREATMENT PLANT | ||||||||||
| 1 | 1.98 | 33.0 | 33.0 | 1 | Temp | −0.038 | 0.544 | −0.419 | 0.642 | −0.339 |
| 2 | 1.72 | 28.6 | 61.6 | 2 | Cond | −0.231 | −0.404 | −0.566 | −0.340 | −0.562 |
| 3 | 1.27 | 21.2 | 82.8 | 3 | DO conc | 0.089 | 0.465 | −0.547 | −0.492 | 0.484 |
| 4 | 0.517 | 8.6 | 91.4 | 4 | pH | −0.233 | −0.508 | −0.386 | 0.465 | 0.568 |
| 5 | 0.46 | 7.7 | 99.1 | 5 | ORP | 0.685 | −0.135 | −0.061 | 0.121 | 0.027 |
| 6 | Free Cl2 | 0.643 | −0.219 | −0.227 | 0.024 | −0.112 | ||||
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sammon, N.B.; Harrower, K.M.; Fabbro, L.D.; Reed, R.H. Incidence and Distribution of Microfungi in a Treated Municipal Water Supply System in Sub-Tropical Australia. Int. J. Environ. Res. Public Health 2010, 7, 1597-1611. https://doi.org/10.3390/ijerph7041597
Sammon NB, Harrower KM, Fabbro LD, Reed RH. Incidence and Distribution of Microfungi in a Treated Municipal Water Supply System in Sub-Tropical Australia. International Journal of Environmental Research and Public Health. 2010; 7(4):1597-1611. https://doi.org/10.3390/ijerph7041597
Chicago/Turabian StyleSammon, Noel B., Keith M. Harrower, Larelle D. Fabbro, and Rob H. Reed. 2010. "Incidence and Distribution of Microfungi in a Treated Municipal Water Supply System in Sub-Tropical Australia" International Journal of Environmental Research and Public Health 7, no. 4: 1597-1611. https://doi.org/10.3390/ijerph7041597
APA StyleSammon, N. B., Harrower, K. M., Fabbro, L. D., & Reed, R. H. (2010). Incidence and Distribution of Microfungi in a Treated Municipal Water Supply System in Sub-Tropical Australia. International Journal of Environmental Research and Public Health, 7(4), 1597-1611. https://doi.org/10.3390/ijerph7041597