Intraoperative Flow Cytometry for the Evaluation of Meningioma Grade
Abstract
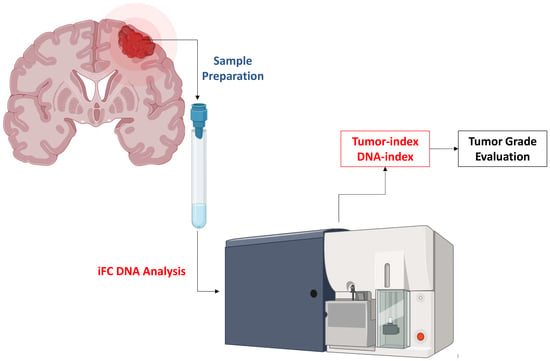
:1. Introduction
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23 (Suppl. S2), iii1–iii105. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Mpairamidis, E.; Psarros, A.; Sfakianos, G.; Prodromou, N. Intracranial meningiomas in children: Report of 8 cases. Pediatr. Neurosurg. 2008, 44, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Stemmer-Rachamimov, A.O.; Niemierko, A.; Larvie, M.; Curry, W.T.; Barker, F.G.; Martuza, R.L.; McGuone, D.; Oh, K.S.; Loeffler, J.S.; et al. Benign meningiomas (WHO Grade I) with atypical histological features: Correlation of histopathological features with clinical outcomes. J. Neurosurg. 2016, 124, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Tamrazi, B.; Shiroishi, M.S.; Liu, C.S. Advanced Imaging of Intracranial Meningiomas. Neurosurg. Clin. N Am. 2016, 27, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Alexiou, G.A.; Vartholomatos, G.; Kobayashi, T.; Voulgaris, S.; Kyritsis, A.P. The emerging role of intraoperative flow cytometry in intracranial tumor surgery. Clin. Neurol. Neurosurg. 2020, 192, 105742. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Vartholomatos, G.; Goussia, A.; Batistatou, A.; Tsamis, K.; Voulgaris, S.; Kyritsis, A.P. Fast cell cycle analysis for intraoperative characterization of brain tumor margins and malignancy. J. Clin. Neurosci. 2015, 22, 129–132. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Basiari, L.; Exarchakos, G.; Kastanioudakis, I.; Komnos, I.; Michali, M.; Markopoulos, G.S.; Batistatou, A.; Papoudou-Bai, A.; Alexiou, G.A. Intraoperative flow cytometry for head and neck lesions. Assessment of malignancy and tumour-free resection margins. Oral. Oncol. 2019, 99, 104344. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Harissis, H.; Andreou, M.; Tatsi, V.; Pappa, L.; Kamina, S.; Batistatou, A.; Markopoulos, G.S.; Alexiou, G.A. Rapid Assessment of Resection Margins During Breast Conserving Surgery Using Intraoperative Flow Cytometry. Clin. Breast Cancer 2021, 21, e602–e610. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Glantzounis, G.K.; Goussia, A.C.; Lianos, G.D.; Karampa, A.; Alexiou, G.A.; Vartholomatos, G. Touch Imprint Intraoperative Flow Cytometry as a Complementary Tool for Detailed Assessment of Resection Margins and Tumor Biology in Liver Surgery for Primary and Metastatic Liver Neoplasms. Methods Protoc. 2021, 4, 66. [Google Scholar] [CrossRef]
- Paliouras, A.; Markopoulos, G.S.; Tsampalas, S.; Mantziou, S.; Giannakis, I.; Baltogiannis, D.; Glantzounis, G.K.; Alexiou, G.A.; Lampri, E.; Sofikitis, N.; et al. Accurate Characterization of Bladder Cancer Cells with Intraoperative Flow Cytometry. Cancers 2022, 14, 5440. [Google Scholar] [CrossRef]
- Anastasiadi, Z.; Mantziou, S.; Akrivis, C.; Paschopoulos, M.; Balasi, E.; Lianos, G.D.; Alexiou, G.A.; Mitsis, M.; Vartholomatos, G.; Markopoulos, G.S. Intraoperative Flow Cytometry for the Characterization of Gynecological Malignancies. Biology 2022, 11, 1339. [Google Scholar] [CrossRef]
- Georvasili, V.K.; Markopoulos, G.S.; Batistatou, A.; Mitsis, M.; Messinis, T.; Lianos, G.D.; Alexiou, G.; Vartholomatos, G.; Bali, C.D. Detection of cancer cells and tumor margins during colorectal cancer surgery by intraoperative flow cytometry. Int. J. Surg. 2022, 104, 106717. [Google Scholar] [CrossRef]
- Alexiou, G.; Vartholomatos, G. Intraoperative Flow Cytometry in Pediatric Brain Tumors. In Pediatric Neurosurgery for Clinicians; Alexiou, G., Prodromou, N., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Vartholomatos, G.; Alexiou, G.A.; Markopoulos, G.S. The Past, Present and Future of Flow Cytometry in Central Nervous System Malignancies. Methods Protoc. 2021, 4, 11. [Google Scholar] [CrossRef]
- Shioyama, T.; Muragaki, Y.; Maruyama, T.; Komori, T.; Iseki, H. Intraoperative flow cytometry analysis of glioma tissue for rapid determination of tumor presence and its histopathological grade: Clinical article. J. Neurosurg. 2013, 118, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Alexiou, G.A.; Vartholomatos, G.; Goussia, A.; Voulgaris, S.; Kyritsis, A.P. Letter: Is Intraoperative Pathology Needed if 5-Aminolevulinic-Acid-Induced Tissue Fluorescence Is Found in Stereotactic Brain Tumor Biopsy? Neurosurgery 2020, 87, E425–E426. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Alexiou, G.A.; Voulgaris, S.; Kyritsis, A.P. Intraoperative Immunophenotypic Analysis for Diagnosis and Classification of Primary Central Nervous System Lymphomas. World Neurosurg. 2018, 117, 464–465. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Alexiou, G.A.; Batistatou, A.; Lykoudis, E.; Voulgaris, S.; Kyritsis, A.P. GV/GA Sarissa-Lancet: A Proposed Real-Time Flow Cytometer for Intraoperative Identification of Glioma Margins. Surg. Innov. 2016, 23, 104–105. [Google Scholar] [CrossRef] [Green Version]
- Markopoulos, G.S.; Goussia, A.; Bali, C.D.; Messinis, T.; Alexiou, G.A.; Vartholomatos, G. Resection Margins Assessment by Intraoperative Flow Cytometry in Pancreatic Cancer. Ann. Surg. Oncol. 2022, 29, 4643–4645. [Google Scholar] [CrossRef]
- Crone, K.R.; Challa, V.R.; Kute, T.E.; Moody, D.M.; Kelly, D.L., Jr. Relationship between flow cytometric features and clinical behavior of meningiomas. Neurosurgery 1988, 23, 720–724. [Google Scholar] [CrossRef]
- Lin, Y.W.; Tai, S.H.; Huang, Y.H.; Chang, C.C.; Juan, W.S.; Chao, L.C.; Wen, M.J.; Hung, Y.C.; Lee, E.J. The application of flow cytometry for evaluating biological aggressiveness of intracranial meningiomas. Cytom. B Clin. Cytom. 2015, 88, 312–319. [Google Scholar] [CrossRef]
- Oya, S.; Yoshida, S.; Tsuchiya, T.; Fujisawa, N.; Mukasa, A.; Nakatomi, H.; Saito, N.; Matsui, T. Intraoperative quantification of meningioma cell proliferation potential using rapid flow cytometry reveals intratumoral heterogeneity. Cancer Med. 2019, 8, 2793–2801. [Google Scholar] [CrossRef] [Green Version]
- Alexiou, A.G.; Zikou, K.A.; Vartholomatos, G.; Goussia, A.; Voulgaris, S.; Kyritsis, A.P.; Argyropoulou, M.I. Correlation of DNA ploidy and cell cycle analysis with diffusion tensor and dynamic susceptibility contrast MRI metrics in meningiomas. Hell. J. Radiol. 2018, 3, 1–6. [Google Scholar]
- Matsuoka, G.; Eguchi, S.; Anami, H.; Ishikawa, T.; Yamaguchi, K.; Nitta, M.; Muragaki, Y.; Kawamata, T. Ultrarapid Evaluation of Meningioma Malignancy by Intraoperative Flow Cytometry. World Neurosurg. 2018, 120, 320–327. [Google Scholar] [CrossRef]
- WHO Classification of Tumours of the Central Nervous System, Editorial Board, 5th ed.; International Agency for Research in Cancer: Lyon, France, 2021.
- Mirian, C.; Duun-Henriksen, A.K.; Juratli, T.; Sahm, F.; Spiegl-Kreinecker, S.; Peyre, M.; Biczok, A.; Tonn, J.-C.; Goutagny, S.; Bertero, L.; et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: An individual patient data meta-analysis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 378–387. [Google Scholar] [CrossRef]
- Guyot, A.; Duchesne, M.; Robert, S.; Lia, A.-S.; Derouault, P.; Scaon, E.; Lemnos, L.; Salle, H.; Durand, K.; Labrousse, F. Analysis of CDKN2A gene alterations in recurrent and non-recurrent meningioma. J. Neurooncol. 2019, 145, 449–459. [Google Scholar] [CrossRef]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.-G.; Mawrin, C.; Ketter, R. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef]



| Grade 1 | Grade 2/3 | |
|---|---|---|
| No of patients (%) | 41 (69.5%) | 18 (30.5%) |
| Diploid (%) | 29 (49.1%) | 9 (15.2%) |
| Aneuploid (%) | 13(22%) | 8 (13.7%) |
| G0/G1 (median) | 92.8% | 82% |
| S-phase (median) | 2% | 4.5% |
| G2/M phase (median) | 4% | 10% |
| Tumor index (S + G2/M) | 7.3% | 16% |
| Ki-67 (median) | 2% | 8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexiou, G.A.; Markopoulos, G.S.; Vartholomatos, E.; Goussia, A.C.; Dova, L.; Dimitriadis, S.; Mantziou, S.; Zoi, V.; Nasios, A.; Sioka, C.; et al. Intraoperative Flow Cytometry for the Evaluation of Meningioma Grade. Curr. Oncol. 2023, 30, 832-838. https://doi.org/10.3390/curroncol30010063
Alexiou GA, Markopoulos GS, Vartholomatos E, Goussia AC, Dova L, Dimitriadis S, Mantziou S, Zoi V, Nasios A, Sioka C, et al. Intraoperative Flow Cytometry for the Evaluation of Meningioma Grade. Current Oncology. 2023; 30(1):832-838. https://doi.org/10.3390/curroncol30010063
Chicago/Turabian StyleAlexiou, George A., Georgios S. Markopoulos, Evrysthenis Vartholomatos, Anna C. Goussia, Lefkothea Dova, Savvas Dimitriadis, Stefania Mantziou, Vasiliki Zoi, Anastasios Nasios, Chrissa Sioka, and et al. 2023. "Intraoperative Flow Cytometry for the Evaluation of Meningioma Grade" Current Oncology 30, no. 1: 832-838. https://doi.org/10.3390/curroncol30010063
APA StyleAlexiou, G. A., Markopoulos, G. S., Vartholomatos, E., Goussia, A. C., Dova, L., Dimitriadis, S., Mantziou, S., Zoi, V., Nasios, A., Sioka, C., Kyritsis, A. P., Voulgaris, S., & Vartholomatos, G. (2023). Intraoperative Flow Cytometry for the Evaluation of Meningioma Grade. Current Oncology, 30(1), 832-838. https://doi.org/10.3390/curroncol30010063