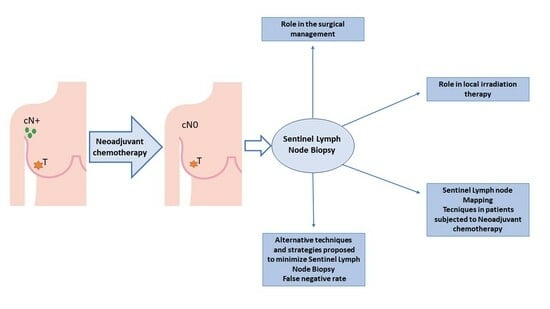
The Role of Sentinel Lymph Node Biopsy in Breast Cancer Patients Who Become Clinically Node-Negative Following Neo-Adjuvant Chemotherapy: A Literature Review
Abstract
:1. Introduction
2. Methods
3. SLNB Role in the Surgical Management of cN+ Patients Resulted cN0 after NACT
3.1. Feasibility, SLNs Identification Rate, and False Negative Rate
3.2. Effect of SLNB Procedures on Local Recurrence
3.3. Effect on Outcome
3.4. Requirements Regarding the Number of SLNs Retrieved
3.5. Effects on Clinical Practice
3.6. Effect on Complications
4. Role of Local Irradiation Therapy after SLNB in cN+ Patients Who Resulted cN0 after NACT
5. State of the Art of SLN Mapping Techniques in Patients Subjected to NACT
6. Alternative Techniques and Strategies Proposed to Minimize SLNB FNR
7. Conclusions
8. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; et al. A Randomized Comparison of Sentinel-Node Biopsy with Routine Axillary Dissection in Breast Cancer. N. Engl. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Viale, G.; Paganelli, G.; Zurrida, S.; Luini, A.; Galimberti, V.; Veronesi, P.; Intra, M.; Maisonneuve, P.; Zucca, F.; et al. Sentinel Lymph Node Biopsy in Breast Cancer: Ten-Year Results of a Randomized Controlled Study. Ann. Surg. 2010, 251, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Hunt, K.K.; Ballman, K.V.; Beitsch, P.D.; Whitworth, P.W.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; McCall, L.M.; Morrow, M. Axillary Dissection vs. No Axillary Dissection in Women with Invasive Breast Cancer and Sentinel Node Metastasis: A Randomized Clinical Trial. JAMA 2011, 305, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs. No Axillary Dissection on 10-Year Overall Survival among Women with Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Tinterri, C.; Sagona, A.; Barbieri, E.; Di Maria Grimaldi, S.; Caraceni, G.; Ambrogi, G.; Jacobs, F.; Biondi, E.; Scardina, L.; Gentile, D. Sentinel Lymph Node Biopsy in Breast Cancer Patients Undergoing Neo-Adjuvant Chemotherapy: Clinical Experience with Node-Negative and Node-Positive Disease Prior to Systemic Therapy. Cancers 2023, 15, 1719. [Google Scholar] [CrossRef]
- Spring, L.M.; Bar, Y.; Isakoff, S.J. The Evolving Role of Neoadjuvant Therapy for Operable Breast Cancer. J. Natl. Compr. Canc. Netw. 2022, 20, 723–734. [Google Scholar] [CrossRef]
- Bonadonna, G.; Veronesi, U.; Brambilla, C.; Ferrari, L.; Luini, A.; Greco, M.; Bartoli, C.; Coopmans de Yoldi, G.; Zucali, R.; Rilke, F. Primary Chemotherapy to Avoid Mastectomy in Tumors with Diameters of Three Centimeters or More. J. Natl. Cancer Inst. 1990, 82, 1539–1545. [Google Scholar] [CrossRef]
- Makris, A.; Powles, T.J.; Ashley, S.E.; Chang, J.; Hickish, T.; Tidy, V.A.; Nash, A.G.; Ford, H.T. A Reduction in the Requirements for Mastectomy in a Randomized Trial of Neoadjuvant Chemoendocrine Therapy in Primary Breast Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1998, 9, 1179–1184. [Google Scholar] [CrossRef]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B.J.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of Preoperative Chemotherapy on Local-Regional Disease in Women with Operable Breast Cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef]
- Kim, T.; Giuliano, A.E.; Lyman, G.H. Lymphatic Mapping and Sentinel Lymph Node Biopsy in Early Stage Breast Carcinoma: A Metaanalysis. Cancer 2006, 106, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Mansel, R.E.; Fallowfield, L.; Kissin, M.; Goyal, A.; Newcombe, R.G.; Dixon, J.M.; Yiangou, C.; Horgan, K.; Bundred, N.; Monypenny, I.; et al. Randomized Multicenter Trial of Sentinel Node Biopsy versus Standard Axillary Treatment in Operable Breast Cancer: The ALMANAC Trial. J. Natl. Cancer Inst. 2006, 98, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Maisonneuve, P.; Gatti, G.; et al. Sentinel-Lymph-Node Biopsy as a Staging Procedure in Breast Cancer: Update of a Randomised Controlled Study. Lancet Oncol. 2006, 7, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; White, L.; Allred, N.; Meyers, M.; Dickson, D.; Dupont, E.; Cantor, A.; Ly, Q.; Dessureault, S.; King, J.; et al. Survival Outcomes in Node-Negative Breast Cancer Patients Evaluated with Complete Axillary Node Dissection versus Sentinel Lymph Node Biopsy. Ann. Surg. Oncol. 2006, 13, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Zavagno, G.; De Salvo, G.L.; Scalco, G.; Bozza, F.; Barutta, L.; Del Bianco, P.; Renier, M.; Racano, C.; Carraro, P.; Nitti, D. A Randomized Clinical Trial on Sentinel Lymph Node Biopsy versus Axillary Lymph Node Dissection in Breast Cancer: Results of the Sentinella/GIVOM Trial. Ann. Surg. 2008, 247, 207–213. [Google Scholar] [CrossRef]
- Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L.; et al. The Value of Semiquantitative Parameters Derived from (18)F-FDG PET/CT for Predicting Response to Neoadjuvant Chemotherapy in a Cohort of Patients with Different Molecular Subtypes of Breast Cancer. Cancers 2022, 14, 5869. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Sirico, M.; Virga, A.; Conte, B.; Urbini, M.; Ulivi, P.; Gianni, C.; Merloni, F.; Palleschi, M.; Gasperoni, M.; Curcio, A.; et al. Neoadjuvant Endocrine Therapy for Luminal Breast Tumors: State of the Art, Challenges and Future Perspectives. Crit. Rev. Oncol. Hematol. 2023, 181, 103900. [Google Scholar] [CrossRef]
- Tadros, A.B.; Yang, W.T.; Krishnamurthy, S.; Rauch, G.M.; Smith, B.D.; Valero, V.; Black, D.M.; Lucci, A.J.; Caudle, A.S.; DeSnyder, S.M.; et al. Identification of Patients with Documented Pathologic Complete Response in the Breast after Neoadjuvant Chemotherapy for Omission of Axillary Surgery. JAMA Surg. 2017, 152, 665–670. [Google Scholar] [CrossRef]
- Caudle, A.S.; Bedrosian, I.; Milton, D.R.; DeSnyder, S.M.; Kuerer, H.M.; Hunt, K.K.; Mittendorf, E.A. Use of Sentinel Lymph Node Dissection after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer at Diagnosis: Practice Patterns of American Society of Breast Surgeons Members. Ann. Surg. Oncol. 2017, 24, 2925–2934. [Google Scholar] [CrossRef]
- Pilewskie, M.; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol. 2017, 3, 549–555. [Google Scholar] [CrossRef]
- Murphy, B.L.; Day, C.N.; Hoskin, T.L.; Habermann, E.B.; Boughey, J.C. Neoadjuvant Chemotherapy Use in Breast Cancer Is Greatest in Excellent Responders: Triple-Negative and HER2+ Subtypes. Ann. Surg. Oncol. 2018, 25, 2241–2248. [Google Scholar] [CrossRef]
- Fisher, C.S.; Margenthaler, J.A.; Hunt, K.K.; Schwartz, T. The Landmark Series: Axillary Management in Breast Cancer. Ann. Surg. Oncol. 2020, 27, 724–729. [Google Scholar] [CrossRef]
- Charfare, H.; Limongelli, S.; Purushotham, A.D. Neoadjuvant Chemotherapy in Breast Cancer. Br. J. Surg. 2005, 92, 14–23. [Google Scholar] [CrossRef]
- Nason, K.S.; Anderson, B.O.; Byrd, D.R.; Dunnwald, L.K.; Eary, J.F.; Mankoff, D.A.; Livingston, R.; Schmidt, R.A.; Jewell, K.D.; Yeung, R.S.; et al. Increased False Negative Sentinel Node Biopsy Rates after Preoperative Chemotherapy for Invasive Breast Carcinoma. Cancer 2000, 89, 2187–2194. [Google Scholar] [CrossRef]
- Pecha, V.; Kolarik, D.; Kozevnikova, R.; Hovorkova, K.; Hrabetova, P.; Halaska, M.; Sottner, O.; Trnkova, M.; Petruzelka, L.; Kolarova, H. Sentinel Lymph Node Biopsy in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Cancer 2011, 117, 4606–4616. [Google Scholar] [CrossRef] [PubMed]
- Canavese, G.; Dozin, B.; Vecchio, C.; Tomei, D.; Villa, G.; Carli, F.; Del Mastro, L.; Levaggi, A.; Rossello, C.; Spinaci, S.; et al. Accuracy of Sentinel Lymph Node Biopsy after Neo-Adjuvant Chemotherapy in Patients with Locally Advanced Breast Cancer and Clinically Positive Axillary Nodes. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2011, 37, 688–694. [Google Scholar] [CrossRef]
- Cohen, L.F.; Breslin, T.M.; Kuerer, H.M.; Ross, M.I.; Hunt, K.K.; Sahin, A.A. Identification and Evaluation of Axillary Sentinel Lymph Nodes in Patients with Breast Carcinoma Treated with Neoadjuvant Chemotherapy. Am. J. Surg. Pathol. 2000, 24, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- AGO Breast Committee. Available online: www.ago-online.de (accessed on 1 June 2023).
- Banys-Paluchowski, M.; de Boniface, J. Axillary Staging in Node-Positive Breast Cancer Converting to Node Negativity through Neoadjuvant Chemotherapy: Current Evidence and Perspectives. Scand. J. Surg. SJS Off. Organ Finn. Surg. Soc. Scand. Surg. Soc. 2023, 112, 117–125. [Google Scholar] [CrossRef]
- Tan, V.K.M.; Goh, B.K.P.; Fook-Chong, S.; Khin, L.-W.; Wong, W.-K.; Yong, W.-S. The Feasibility and Accuracy of Sentinel Lymph Node Biopsy in Clinically Node-Negative Patients after Neoadjuvant Chemotherapy for Breast Cancer--a Systematic Review and Meta-Analysis. J. Surg. Oncol. 2011, 104, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Brown, A.; Anderson, S.; Smith, R.; Julian, T.; Miller, B.; Bear, H.D.; Caldwell, C.B.; Walker, A.P.; Mikkelson, W.M.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Foy, M.; Cox, D.D.; Kuerer, H.M.; Hunt, K.K.; Cormier, J.N. Meta-Analysis of Sentinel Lymph Node Biopsy after Preoperative Chemotherapy in Patients with Breast Cancer. Br. J. Surg. 2006, 93, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Terribile, D.; Franco, A.; Martullo, A.; Orlandi, A.; Magno, S.; Di Leone, A.; Moschella, F.; Natale, M.; D’Archi, S.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer: Preliminary Experience with Clinically Node Negative Patients after Systemic Treatment. J. Pers. Med. 2021, 11, 172. [Google Scholar] [CrossRef]
- Shirzadi, A.; Mahmoodzadeh, H.; Qorbani, M. Assessment of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy for Breast Cancer in Two Subgroups: Initially Node Negative and Node Positive Converted to Node Negative—A Systemic Review and Meta-Analysis. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2019, 24, 18. [Google Scholar] [CrossRef]
- Cavalcante, F.P.; Millen, E.C.; Novita, G.G.; Zerwes, F.P.; Mattar, A.; Machado, R.H.S.; Frasson, A.L. Sentinel Lymph Node Biopsy Following Neoadjuvant Chemotherapy: An Evidence-Based Review and Recommendations for Current Practice. Chin. Clin. Oncol. 2023, 12, 6. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel Lymph Node Surgery after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer: The ACOSOG Z1071 (Alliance) Clinical Trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-Lymph-Node Biopsy in Patients with Breast Cancer before and after Neoadjuvant Chemotherapy (SENTINA): A Prospective, Multicentre Cohort Study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boileau, J.-F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: The SN FNAC Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients with Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef]
- Kuemmel, S.; Heil, J.; Rueland, A.; Seiberling, C.; Harrach, H.; Schindowski, D.; Lubitz, J.; Hellerhoff, K.; Ankel, C.; Graßhoff, S.-T.; et al. A Prospective, Multicenter Registry Study to Evaluate the Clinical Feasibility of Targeted Axillary Dissection (TAD) in Node-Positive Breast Cancer Patients. Ann. Surg. 2022, 276, e553–e562. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, P.K.; Tayeh, S.; Michell, M.J.; Mokbel, K. The Evolving Role of Marked Lymph Node Biopsy (MLNB) and Targeted Axillary Dissection (TAD) after Neoadjuvant Chemotherapy (NACT) for Node-Positive Breast Cancer: Systematic Review and Pooled Analysis. Cancers 2021, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Žatecký, J.; Coufal, O.; Zapletal, O.; Kubala, O.; Kepičová, M.; Faridová, A.; Rauš, K.; Gatěk, J.; Kosáč, P.; Peteja, M. Ideal Marker for Targeted Axillary Dissection (IMTAD): A Prospective Multicentre Trial. World J. Surg. Oncol. 2023, 21, 252. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Kühn, T.; Masannat, Y.; Rubio, I.; de Boniface, J.; Ditsch, N.; Karadeniz Cakmak, G.; Karakatsanis, A.; Dave, R.; Hahn, M.; et al. Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/IBRA-NET, NCT 05559411). Cancers 2023, 15, 1173. [Google Scholar] [CrossRef]
- Kahler-Ribeiro-Fontana, S.; Pagan, E.; Magnoni, F.; Vicini, E.; Morigi, C.; Corso, G.; Intra, M.; Canegallo, F.; Ratini, S.; Leonardi, M.C.; et al. Long-Term Standard Sentinel Node Biopsy after Neoadjuvant Treatment in Breast Cancer: A Single Institution Ten-Year Follow-Up. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 804–812. [Google Scholar] [CrossRef]
- Barrio, A.V.; Montagna, G.; Mamtani, A.; Sevilimedu, V.; Edelweiss, M.; Capko, D.; Cody, H.S., 3rd; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.; et al. Nodal Recurrence in Patients with Node-Positive Breast Cancer Treated with Sentinel Node Biopsy Alone after Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol. 2021, 7, 1851–1855. [Google Scholar] [CrossRef]
- Martelli, G.; Barretta, F.; Miceli, R.; Folli, S.; Maugeri, I.; Listorti, C.; Scaperrotta, G.; Baili, P.; Pruneri, G.; Capri, G.; et al. Sentinel Node Biopsy Alone or with Axillary Dissection in Breast Cancer Patients after Primary Chemotherapy: Long-Term Results of a Prospective Interventional Study. Ann. Surg. 2022, 276, e544–e552. [Google Scholar] [CrossRef] [PubMed]
- Piltin, M.A.; Hoskin, T.L.; Day, C.N.; Davis, J.J.; Boughey, J.C. Oncologic Outcomes of Sentinel Lymph Node Surgery after Neoadjuvant Chemotherapy for Node-Positive Breast Cancer. Ann. Surg. Oncol. 2020, 27, 4795–4801. [Google Scholar] [CrossRef]
- Galimberti, V.; Ribeiro Fontana, S.K.; Vicini, E.; Morigi, C.; Sargenti, M.; Corso, G.; Magnoni, F.; Intra, M.; Veronesi, P. This House Believes That: Sentinel Node Biopsy Alone Is Better than TAD after NACT for CN+ Patients. Breast 2023, 67, 21–25. [Google Scholar] [CrossRef]
- Eubreast.Com Home. Available online: https://www.eubreast.com/?Trials/AXSANA (accessed on 1 June 2023).
- Banys-Paluchowski, M.; Gasparri, M.L.; de Boniface, J.; Gentilini, O.; Stickeler, E.; Hartmann, S.; Thill, M.; Rubio, I.T.; Di Micco, R.; Bonci, E.-A.; et al. Surgical Management of the Axilla in Clinically Node-Positive Breast Cancer Patients Converting to Clinical Node Negativity through Neoadjuvant Chemotherapy: Current Status, Knowledge Gaps, and Rationale for the EUBREAST-03 AXSANA Study. Cancers 2021, 13, 1565. [Google Scholar] [CrossRef]
- Ogawa, Y.; Ikeda, K.; Watanabe, C.; Kamei, Y.; Tokunaga, S.; Tsuboguchi, Y.; Inoue, T.; Fukushima, H.; Ichiki, M. Sentinel Node Biopsy for Axillary Management after Neoadjuvant Therapy for Breast Cancer: A Single-Center Retrospective Analysis with Long Follow-Up. Surg. Today 2018, 48, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Classe, J.-M.; Loaec, C.; Gimbergues, P.; Alran, S.; de Lara, C.T.; Dupre, P.F.; Rouzier, R.; Faure, C.; Paillocher, N.; Chauvet, M.P.; et al. Sentinel Lymph Node Biopsy without Axillary Lymphadenectomy after Neoadjuvant Chemotherapy Is Accurate and Safe for Selected Patients: The GANEA 2 Study. Breast Cancer Res. Treat. 2019, 173, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, I.; Alsharif, E.; Park, S.; Kim, J.-M.; Ryu, J.M.; Nam, S.J.; Kim, S.W.; Yu, J.; Lee, S.K.; et al. Use of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer in Diagnosis. J. Breast Cancer 2018, 21, 433–441. [Google Scholar] [CrossRef]
- Berberoglu, K.; Erdemir, A.; Rasa, K.; Baloglu, H.; Cakmakci, M. Role of Gamma Probe-Assisted Intraoperative Sentinel Lymph Node Evaluation in Predicting Axillary Breast Cancer Metastasis after Neoadjuvant Chemotherapy. Nucl. Med. Commun. 2020, 41, 120–125. [Google Scholar] [CrossRef]
- Jimenez-Gomez, M.; Loro-Pérez, J.; Vega-Benítez, V.; Hernández-Hernández, J.R.; Aguirre, N.A. Axillary Management in Patients with Breast Cancer and Positive Axilla at Diagnosis. Experience in a Spanish University Hospital with a 5-Year Follow-Up. J. Cancer Res. Ther. 2023, 19, 183–190. [Google Scholar] [CrossRef]
- Wong, S.M.; Basik, M.; Florianova, L.; Margolese, R.; Dumitra, S.; Muanza, T.; Carbonneau, A.; Ferrario, C.; Boileau, J.F. Oncologic Safety of Sentinel Lymph Node Biopsy Alone after Neoadjuvant Chemotherapy for Breast Cancer. Ann. Surg. Oncol. 2021, 28, 2621–2629. [Google Scholar] [CrossRef]
- Riogi, B.; Sripadam, R.; Barker, D.; Harris, O.; Innes, H.; Chagla, L. Management of the Axilla Following Neoadjuvant Chemotherapy for Breast Cancer- A Change in Practice. Surgeon 2021, 19, 1–7. [Google Scholar] [CrossRef]
- Damin, A.P.; Zancan, M.; Melo, M.P.; Biazus, J. V Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer: Guiding a More Selective Axillary Approach. Breast Cancer Res. Treat. 2021, 186, 527–534. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, H.; Kim, J.; Chung, I.Y.; Kim, H.J.; Ko, B.S.; Lee, J.W.; Ahn, S.H.; Son, B.H. Prognosis According to Clinical and Pathologic Lymph Node Status in Breast Cancer Patients Who Underwent Sentinel Lymph Node Biopsy Alone after Neoadjuvant Therapy. PLoS ONE 2021, 16, e0251597. [Google Scholar] [CrossRef]
- Cabıoğlu, N.; Karanlık, H.; Yıldırım, N.; Müslümanoğlu, M.; Çakmak Karadeniz, G.; Trabulus Can, D.; Tükenmez, M.; Ersoy, Y.E.; Uras, C.; Zengel, B.; et al. Favorable Outcome with Sentinel Lymph Node Biopsy Alone after Neoadjuvant Chemotherapy in Clinically Node Positive Breast Cancer at Diagnosis: Turkish Multicentric NEOSENTI-TURK MF-18-02-Study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 2506–2514. [Google Scholar] [CrossRef]
- Galimberti, V.; Ribeiro Fontana, S.K.; Maisonneuve, P.; Steccanella, F.; Vento, A.R.; Intra, M.; Naninato, P.; Caldarella, P.; Iorfida, M.; Colleoni, M.; et al. Sentinel Node Biopsy after Neoadjuvant Treatment in Breast Cancer: Five-Year Follow-up of Patients with Clinically Node-Negative or Node-Positive Disease before Treatment. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2016, 42, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Tercan, I.C.; Zengel, B.; Ozdemir, O.; Cavdar, D.; Tasli, F.; Adibelli, Z.H.; Karatas, M.; Simsek, C.; Durusoy, I.R.; Uslu, A. The Oncologic Safety of Sentinel Lymph Node Biopsy in Patients with Node-Positive Breast Cancer with Complete Response to Neoadjuvant Chemotherapy: A Single-Center Experience. Breast J. 2023, 2023, 4549033. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, J.; Kim, S.-Y.; Lee, E.S.; Kang, H.-S.; Lee, S.; Jung, S.-Y.; Lee, E. Sentinel Lymph Node Biopsy in Breast Cancer Patients with Pathological Complete Response in the Axillary Lymph Node after Neoadjuvant Chemotherapy. J. Breast Cancer 2021, 24, 531–541. [Google Scholar] [CrossRef]
- Sharp, N.E.; Sachs, D.B.; Melchior, N.M.; Albaneze, P.; Nardello, S.; Sigurdson, E.R.; Deng, M.; Aggon, A.A.; Daly, J.M.; Bleicher, R.J. Does the False-Negative Rate for 1 or 2 Negative Sentinel Nodes after Neo-Adjuvant Chemotherapy Translate into a High Local Recurrence Rate? Breast J. 2021, 27, 335–344. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Hoskin, T.L.; Day, C.N.; Degnim, A.C.; Jakub, J.W.; Hieken, T.J.; Boughey, J.C. Decreasing Use of Axillary Dissection in Node-Positive Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2018, 25, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.A.; Lehman, C.D.; Harris, J.R.; Pockaj, B.A.; Khouri, N.; Hylton, N.F.; Miller, M.J.; Whelan, T.; Pierce, L.J.; Esserman, L.J.; et al. Statement of the Science Concerning Locoregional Treatments after Preoperative Chemotherapy for Breast Cancer: A National Cancer Institute Conference. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or Surgery of the Axilla after a Positive Sentinel Node in Breast Cancer (EORTC 10981-22023 AMAROS): A Randomised, Multicentre, Open-Label, Phase 3 Non-Inferiority Trial. Lancet Oncol. 2014, 15, 1303–1310. [Google Scholar] [CrossRef]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Miyashita, M.; Niikura, N.; Kumamaru, H.; Miyata, H.; Iwamoto, T.; Kawai, M.; Anan, K.; Hayashi, N.; Aogi, K.; Ishida, T.; et al. Role of Postmastectomy Radiotherapy after Neoadjuvant Chemotherapy in Breast Cancer Patients: A Study from the Japanese Breast Cancer Registry. Ann. Surg. Oncol. 2019, 26, 2475–2485. [Google Scholar] [CrossRef]
- Jagsi, R.; Griffith, K.A.; Vicini, F.; Boike, T.; Burmeister, J.; Dominello, M.M.; Grills, I.; Hayman, J.A.; Moran, J.M.; Paximadis, P.; et al. Toward Improving Patients’ Experiences of Acute Toxicity from Breast Radiotherapy: Insights from the Analysis of Patient-Reported Outcomes in a Large Multicenter Cohort. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4019–4029. [Google Scholar] [CrossRef]
- Hill-Kayser, C.E.; Vachani, C.; Hampshire, M.K.; Di Lullo, G.A.; Metz, J.M. Cosmetic Outcomes and Complications Reported by Patients Having Undergone Breast-Conserving Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 839–844. [Google Scholar] [CrossRef] [PubMed]
- van Hemert, A.K.E.; van Olmen, J.P.; Boersma, L.J.; Maduro, J.H.; Russell, N.S.; Tol, J.; Engelhardt, E.G.; Rutgers, E.J.T.; Vrancken Peeters, M.-J.T.F.D.; van Duijnhoven, F.H. De-ESCAlating RadioTherapy in Breast Cancer Patients with Pathologic Complete Response to Neoadjuvant Systemic Therapy: DESCARTES Study. Breast Cancer Res. Treat. 2023, 199, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Park, W.; Choi, D.H.; Kim, Y.B.; Kim, J.H.; Kim, S.S.; Kim, K.; Kim, J.H.; Ahn, S.-J.; Lee, S.Y.; et al. The Benefit of Post-Mastectomy Radiotherapy in YpN0 Patients after Neoadjuvant Chemotherapy According to Molecular Subtypes. J. Breast Cancer 2019, 22, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.H.; Kim, I.A.; Chang, J.H.; Shin, K.H. Impact of Postmastectomy Radiation Therapy on Breast Cancer Patients According to Pathologic Nodal Status after Modern Neoadjuvant Chemotherapy. Cancer Res. Treat. 2023, 55, 592–602. [Google Scholar] [CrossRef] [PubMed]
- NCT01872975—A Randomized Phase III Clinical Trial Evaluating Post-Mastectomy Chestwall and Regional Nodal XRT and Post-Lumpectomy Regional Nodal XRT in Patients with Positive Axillary Nodes before Neoadjuvant Chemotherapy Who Convert to Pathologically N. Available online: https://www.clinicaltrials.gov/study/NCT01872975 (accessed on 1 June 2023).
- NCT01901094—A Randomized Phase III Trial Comparing Axillary Lymph Node Dissection to Axillary Radiation in Breast Cancer Patients (CT1-3 N1) Who Have Positive Sentinel Lymph Node Disease after Neoadjuvant Chemotherapy. Available online: https://www.clinicaltrials.gov/study/NCT01901094 (accessed on 1 June 2023).
- Ahmed, M.; Purushotham, A.D.; Douek, M. Novel Techniques for Sentinel Lymph Node Biopsy in Breast Cancer: A Systematic Review. Lancet Oncol. 2014, 15, e351–e362. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Chen, B.; Bao, Y.; Luo, C.; Luo, Y.; Li, T.; Lv, J.; Cheng, X. Sentinel Lymph Node Biopsy Mapped with Carbon Nanoparticle Suspensions in Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 818812. [Google Scholar] [CrossRef]
- Rocco, N.; Velotti, N.; Pontillo, M.; Vitiello, A.; Berardi, G.; Accurso, A.; Masone, S.; Musella, M. New Techniques versus Standard Mapping for Sentinel Lymph Node Biopsy in Breast Cancer: A Systematic Review and Meta-Analysis. Updates Surg. 2023, 75, 1699–1710. [Google Scholar] [CrossRef]
- Lorek, A.; Steinhof-Radwańska, K.; Zarębski, W.; Lorek, J.; Stojčev, Z.; Zych, J.; Syrkiewicz, A.; Niemiec, P.; Szyluk, K. Comparative Analysis of Postoperative Complications of Sentinel Node Identification Using the SentiMag(®) Method and the Use of a Radiotracer in Patients with Breast Cancer. Curr. Oncol. 2022, 29, 2887–2894. [Google Scholar] [CrossRef]
- Chirappapha, P.; Chatmongkonwat, T.; Lertsithichai, P.; Pipatsakulroj, W.; Sritara, C.; Sukarayothin, T. Sentinel Lymph Node Biopsy after Neoadjuvant Treatment of Breast Cancer Using Blue Dye, Radioisotope, and Indocyanine Green: Prospective Cohort Study. Ann. Med. Surg. 2020, 59, 156–160. [Google Scholar] [CrossRef]
- Giménez-Climent, J.; Marín-Hernández, C.; Fuster-Diana, C.A.; Torró-Richart, J.A.; Navarro-Cecilia, J. Sentinel Lymph Node Biopsy in Breast Cancer after Neoadjuvant Therapy Using a Magnetic Tracer versus Standard Technique: A Multicentre Comparative Non-Inferiority Study (IMAGINE-II). Int. J. Surg. Open 2021, 35, 4–10. [Google Scholar] [CrossRef]
- Sun, S.; Bai, J.; Wang, X. Comparative Observation of Common Tracers in Sentinel Lymph Node Biopsy of Breast Cancer and a Study on Simplifying Its Surgical Procedure. Front. Surg. 2023, 10, 1180919. [Google Scholar] [CrossRef] [PubMed]
- Donker, M.; Straver, M.E.; Wesseling, J.; Loo, C.E.; Schot, M.; Drukker, C.A.; van Tinteren, H.; Sonke, G.S.; Rutgers, E.J.T.; Vrancken Peeters, M.-J.T.F.D. Marking Axillary Lymph Nodes with Radioactive Iodine Seeds for Axillary Staging after Neoadjuvant Systemic Treatment in Breast Cancer Patients: The MARI Procedure. Ann. Surg. 2015, 261, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Reimer, T.; Gerber, B.; Stubert, J.; Stengel, B.; Stachs, A. Wire Localization of Clip-Marked Axillary Lymph Nodes in Breast Cancer Patients Treated with Primary Systemic Therapy. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018, 44, 1307–1311. [Google Scholar] [CrossRef]
- Hartmann, S.; Stachs, A.; Gerber, B.; Knauerhase, H.; Kamin, F.; Kundt, G.; Reimer, T. Lost Clips after Targeted Lymph Node Biopsy in Breast Cancer Patients: Follow-up of the CLIP-Study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Kühn, T.; de Boniface, J.; Stachs, A.; Winckelmann, A.; Frisell, J.; Wiklander-Bråkenhielm, I.; Stubert, J.; Gerber, B.; Reimer, T. Carbon Tattooing for Targeted Lymph Node Biopsy after Primary Systemic Therapy in Breast Cancer: Prospective Multicentre TATTOO Trial. Br. J. Surg. 2021, 108, 302–307. [Google Scholar] [CrossRef]
- de Boniface, J.; Frisell, J.; Bergkvist, L.; Andersson, Y. Ten-Year Report on Axillary Recurrence after Negative Sentinel Node Biopsy for Breast Cancer from the Swedish Multicentre Cohort Study. Br. J. Surg. 2017, 104, 238–247. [Google Scholar] [CrossRef]
- Natsiopoulos, I.; Intzes, S.; Liappis, T.; Zarampoukas, K.; Zarampoukas, T.; Zacharopoulou, V.; Papazisis, K. Axillary Lymph Node Tattooing and Targeted Axillary Dissection in Breast Cancer Patients Who Presented as CN+ Before Neoadjuvant Chemotherapy and Became CN0 after Treatment. Clin. Breast Cancer 2019, 19, 208–215. [Google Scholar] [CrossRef]
- van Nijnatten, T.J.A.; Simons, J.M.; Smidt, M.L.; van der Pol, C.C.; van Diest, P.J.; Jager, A.; van Klaveren, D.; Kam, B.L.R.; Lobbes, M.B.I.; de Boer, M.; et al. A Novel Less-Invasive Approach for Axillary Staging after Neoadjuvant Chemotherapy in Patients with Axillary Node-Positive Breast Cancer by Combining Radioactive Iodine Seed Localization in the Axilla with the Sentinel Node Procedure (RISAS): A Dutch Pro. Clin. Breast Cancer 2017, 17, 399–402. [Google Scholar] [CrossRef]
- Simons, J.M.; van Nijnatten, T.J.A.; van der Pol, C.C.; van Diest, P.J.; Jager, A.; van Klaveren, D.; Kam, B.L.R.; Lobbes, M.B.I.; de Boer, M.; Verhoef, C.; et al. Diagnostic Accuracy of Radioactive Iodine Seed Placement in the Axilla with Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Node-Positive Breast Cancer. JAMA Surg. 2022, 157, 991–999. [Google Scholar] [CrossRef]
- Lee, C.; Bhatt, A.; Felmlee, J.P.; Trester, P.; Lanners, D.; Paulsen, A.; Brunette, J. How to Safely Perform Magnetic Resonance Imaging-Guided Radioactive Seed Localizations in the Breast. J. Clin. Imaging Sci. 2020, 10, 19. [Google Scholar] [CrossRef]
- Hayes, M.K. Update on Preoperative Breast Localization. Radiol. Clin. N. Am. 2017, 55, 591–603. [Google Scholar] [CrossRef]
- Mango, V.L.; Wynn, R.T.; Feldman, S.; Friedlander, L.; Desperito, E.; Patel, S.N.; Gomberawalla, A.; Ha, R. Beyond Wires and Seeds: Reflector-Guided Breast Lesion Localization and Excision. Radiology 2017, 284, 365–371. [Google Scholar] [CrossRef]
- Sun, J.; Henry, D.A.; Carr, M.J.; Yazdankhahkenary, A.; Laronga, C.; Lee, M.C.; Hoover, S.J.; Sun, W.; Czerniecki, B.J.; Khakpour, N.; et al. Feasibility of Axillary Lymph Node Localization and Excision Using Radar Reflector Localization. Clin. Breast Cancer 2021, 21, e189–e193. [Google Scholar] [CrossRef]
- Simons, J.M.; Scoggins, M.E.; Kuerer, H.M.; Krishnamurthy, S.; Yang, W.T.; Sahin, A.A.; Shen, Y.; Lin, H.; Bedrosian, I.; Mittendorf, E.A.; et al. Prospective Registry Trial Assessing the Use of Magnetic Seeds to Locate Clipped Nodes after Neoadjuvant Chemotherapy for Breast Cancer Patients. Ann. Surg. Oncol. 2021, 28, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Reitsamer, R.; Peintinger, F.; Forsthuber, E.; Sir, A. The Applicability of Magseed® for Targeted Axillary Dissection in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Breast 2021, 57, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, H.I.; Wong, J.M.; Mukhtar, R.A.; Alvarado, M.D.; Price, E.R. Feasibility of Magnetic Seeds for Preoperative Localization of Axillary Lymph Nodes in Breast Cancer Treatment. AJR. Am. J. Roentgenol. 2019, 213, 953–957. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, J.L.; Benjumeda-Gonzalez, A.M.; Amerigo-Góngora, M.; Landra-Dulanto, P.J.; Gonzalez-Corena, Y.; Gomez-Menchero, J. Targeted Axillary Dissection in Breast Cancer by Marking Lymph Node Metastasis with a Magnetic Seed before Starting Neoadjuvant Treatment. J. Surg. Case Rep. 2019, 2019, rjz344. [Google Scholar] [CrossRef]
- Ditsch, N.; Rubio, I.T.; Gasparri, M.L.; de Boniface, J.; Kuehn, T. Breast and Axillary Surgery in Malignant Breast Disease: A Review Focused on Literature of 2018 and 2019. Curr. Opin. Obstet. Gynecol. 2020, 32, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H. Safety of Neoadjuvant Chemotherapy for the Treatment of Breast Cancer. Expert Opin. Drug Saf. 2019, 18, 817–827. [Google Scholar] [CrossRef]
- Man, V.; Kwong, A. Different Strategies in Marking Axillary Lymph Nodes in Breast Cancer Patients Undergoing Neoadjuvant Medical Treatment: A Systematic Review. Breast Cancer Res. Treat. 2021, 186, 607–615. [Google Scholar] [CrossRef]

| Author | Year | Country | Study Design | Number of Involved Centers | Funding Sources |
|---|---|---|---|---|---|
| Choi et al. [55] | 2018 | South Korea | P | Single | No |
| Nguyen et al. [67] | 2018 | USA | R | Single | No |
| Ogawa et al. [53] | 2018 | Japan | R | Single | No |
| Classe et al. [54] | 2019 | France | P | Multicentric | Yes |
| Berberoglu et al. [56] | 2020 | USA | R | Single | None declared |
| Piltin et al. [49] | 2020 | USA | R | Single | None declared |
| Barrio et al. [47] | 2021 | USA | R | Single | Yes |
| Cabioğlu et al. [60] | 2021 | Turkey | R | Multicentric | Yes |
| Damin et al. [60] | 2021 | Brazil | R | Single | No |
| Kahler-Ribeiro-Fontana et al. [41] | 2021 | Italy | R | Single | Yes |
| Kim et al. [65] | 2021 | South Korea | R | Single | No |
| Lee et al. [61] | 2021 | South Korea | R | Single | Yes |
| Riogi et al. [59] | 2021 | UK | P | Single | No |
| Sharp et al. [66] | 2021 | USA | R | Single | Yes |
| Wong et al. [58] | 2021 | Canada | R | Single | None declared |
| Martelli et al. [48] | 2022 | Italy | P | Single | None declared |
| Tercan et al. [64] | 2022 | Turkey | R | Single | None declared |
| Galimberti et al. [50] | 2023 | Italy | R | Single | No |
| Jimenez-Gomez et al. [59] | 2023 | Spain | R | Single | No |
| Tinterri et al. [5] | 2023 | Italy | R | Single | No |
| Author | Enrollment | Patient Stage | N. of cN+ Patients → cN0 after NACT | N. of SLNB Alone | Mean Age (Years) | Axillary Staging | SLN Mapping Technique | Median/Mean of Retrieved Nodes | Median FU (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Choi et al. [55] | 2007–2014 | cT1-T4, N1-3 | 506 | 85 | 44.4 ± 9.3 | SLNB | RI and BD | 5 (2–9) | 51 |
| Nguyen et al. [67] | 2009–2017 | cT0-T4, N1 | 430 | 93 | 50.5 | SLNB | RI and BD | 2 (1–9) | 9 |
| Ogawa et al. [53] | 2006–2015 | cT1-T4, N0-3 | 48 | 33 | 52.6 | SLNB | BD | 2.4 | 59 |
| Classe et al. [54] | 2010–2014 | cT1-4, N0-N2 | 351 | 1 | 52 | SLNB | RI and BD | 2 (1–8) | 36 |
| Berberoglu et al. [56] | / | cT0-4, N0-N2 | 91 | 87 lesions | 47 | SLNB | RI | 1.0–4.0 | |
| Piltin et al. [49] | 2009–2019 | cT1-4, N1-3 | 602 | 159 | 45 | SLNB | Not specified | 3 (1–12) | 34 |
| Barrio et al. [47] | 2013–2019 | cT1-3, N1 | 555 | 234 | 49 | SLNB | RI and BD | 4 (3–5) | 40 |
| Cabioğlu et al. [60] | 2004–2018 | cT1-4, N1-N3 | 303 | 46 | SLNB | RI and BD | 3 | 36 | |
| Damin et al. [60] | 2010–2016 | cT1-4, N1-N2 | 59 | 38 | 49.08 ± 0.84 | SLNB | RI and BD | 2 | 55.8 |
| Kahler-Ribeiro-Fontana et al. [41] | 2000–2015 | cT1-3, N0-N2 | 222 | 131 | 45 | SLNB | RI | 2 (1–6) | 110 |
| Kim et al. [65] | 2006–2015 | cT1-4, N1-3 | 223 | 94 | 46 | SLNB | RI and BD | 2.2 ± 1.2 | 57 |
| Lee et al. [61] | 2003–2014 | cT1-T4, N1-3 | 242 | 760 | 45.1 | SLNB | RI | 4.9 ± 2.6 | 60 |
| Riogi et al. [59] | 2007–2016 | cN0-N+ | 56 | 40 | 50 | SLNB | RI and BD | 2 (1–7) | |
| Sharp et al. [66] | 2004–2018 | cT1-3, N0-N2 | 68 | 68 | 50 | SLNB | RI and BD | 1- ≥3 | 46.8 |
| Wong et al. [58] | 2013–2018 | cT1-3, N0-N2 | 132 | 102 | 50 | SLNB | RI and BD | 3 (2–4) | 36 |
| Martelli et al. [48] | 2007–2015 | cT2, N0-N1 | 121 | 81 | 47 | SLNB | RI | 2 (1–8) | 108 |
| Tercan et al. [64] | 2013–2020 | cT1-4, N1-2 | 90 | 44 (39 ypCR + 6 ypNCR) | 49.6 | SLNB | RI and BD | ≥3 | 34 ± 18 |
| Galimberti et al. [50] | / | cT1-3, N0-N2 | 222 | 222 | 45 | SLNB | RI | 2 (1–6) | 120 |
| Jimenez-Gomez et al. [59] | 2010–2017 | cT1b-T4, N+ | 168 | 48 | SLNB | RI and BD | ≥2 | 60 | |
| Tinterri et al. [5] | 2008–2021 | cT1-4, N0-N+ | 160 | 100 | 50 | SLNB | RI | 1 | 50 |
| Author | Aim of the Study | SNIR (%) | FNR (%) | OS (%) | DFS (%) | Axillary Recurrence (%) | Outcome |
|---|---|---|---|---|---|---|---|
| Choi et al. [55] | Evaluate feasibility of SLNB | 98.3 | 7.5 | 92.9 | 81.2 | 2.0 | SLNB can be feasible and oncologically safe |
| Nguyen et al. [67] | Evaluate effect of SLNB in clinical practice | / | 5 | / | / | 0.0 | Significantly increased use of SLNB alone |
| Ogawa et al. [53] | Assess effectiveness, SNIR, and FNR of SLNB | 94.3 | / | 80.0 | 60.0 | 30.0 | SLNB does not affect the axillary failure rate or the prognosis |
| Classe et al. [54] | Assess diagnostic accuracy and safety of SLNB | / | 11.9 | / | / | / | For SLNB alone, an accurate selection of post-NACT negative SLN patients is necessary |
| Berberoglu et al. [56] | Evaluate diagnostic value of SLNB | 92.6 | 5.7 | / | / | / | SLNB is feasible and efficient |
| Piltin et al. [49] | Compare SLNB alone vs. ALND | / | 3.8 | / | 97.4 | 0.9 | SLNB alone is not oncologically inferior to ALND during a short-term FU period |
| Barrio et al. [47] | Assess axillary LN recurrence | / | / | / | 92.7 | 1 | If ≥3 negative SLNs with SLNB alone, axillary LRR is low |
| Cabioğlu et al. [60] | Evaluate factors affecting local recurrence and overall outcome | / | / | / | 88.0 | 1.1 | ALND could be avoided in selected patients |
| Damin et al. [60] | Evaluate safety of SLNB, efficacy, and oncological outcomes | 93.2 | <10 | 89.0 | 82.0 | 2.6 | SLNB could be successfully used and does not compromise disease control and oncological outcomes |
| Kahler-Ribeiro-Fontana et al. [46] | Assess axillary LN recurrence, OS, DFS | / | / | 84.8 | 81.4 | 1.6 | SLNB alone is acceptable and not associated with a worse outcome |
| Kim et al. [65] | Evaluate safety, axillary LN recurrence rate, and incidence of side effects | / | 10 | 96.3 | 94.2 | 1.1 | SLNB is oncologically safe |
| Lee et al. [61] | Evaluate prognosis and oncological outcomes of SLNB alone | / | / | 93.0 | 98.0 | 2.0 | SLNB alone is associated with low LRR |
| Riogi et al. [59] | Evaluate management of the axilla and outcomes | / | / | 79.4 (of 165 pts) | 24.0 (of 165 pts) | 0.0 | Acceptable outcomesof conservative approach in the axilla after NACT |
| Sharp et al. [66] | Assess LRR rate for SLNB | / | / | 85.0 | 85.0 | 3.0 | Low LRR events and DFS statistically similar between SLNs number |
| Wong et al. [58] | Evaluate oncological safety of SLNB | 96.9 | / | / | / | 5.9 | SLNB alone is associated with low and acceptable short-term axillary recurrence rates |
| Martelli et al. [48] | Assess feasibility of SLNB, OS, DFS | / | / | 89.0 | 79.0 | 0.0 | SLNB is oncologically safe |
| Tercan et al. [64] | Evaluate efficiency and safety of SLNB | / | / | 92.3 in ypCR and 100 in ypNCR | / | 0.0 | No event developed in cases with ypCR and ypNCR in the breast and axilla |
| Galimberti et al. [50] | Assess axillary LN recurrence, incidence of distant events | / | / | / | / | 1.8 | SLNB alone demonstrate no worse outcome in cN+ patients who became cN0 after NACT |
| Jimenez-Gomez et al. [59] | Evaluate feasibility and diagnostic accuracy of SLNB | / | 7 | / | 41.4 | <1 | SLNB provides useful and reliable information about cancer staging, leading to a decrease in possible arm morbidity |
| Tinterri et al. [5] | Compare the characteristics and oncological outcomes of SLNB in cN0 and cN+ patients before NACT and axillary surgery | / | / | 93.2 | 83.6 | 1.3 | SLNB shows good prognosis and low axillary failure rates in cN0 or cN+ patients undergoing NACT who subsequently remained or became cN0 |
| Author | Year | Patients | SLNB Mapping Technique | Detection Rate | Comments |
|---|---|---|---|---|---|
| Chirappapha et al. [83] | 2020 | 21 | RI BD ICG | 53.87% 81.78% 93.22% | Every combination demonstrated a good performance. |
| Giménez-Climent et al. [84] | 2021 | 89 | RI SPIO | 97.8% 97.8% | This study demonstrated a non-inferiority of SPIO compared to RI. |
| Sun et al. [85] | 2023 | 123 (2 patients after NACT) | CNPs CNPs plus BD BD plus ICG | 97.4% 97.6% 95.5% | This study proved that, despite the lack of patients treated with NACT, these techniques could be valid even in this setting of patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrarazzo, G.; Nieri, A.; Firpo, E.; Rattaro, A.; Mignone, A.; Guasone, F.; Manzara, A.; Perniciaro, G.; Spinaci, S. The Role of Sentinel Lymph Node Biopsy in Breast Cancer Patients Who Become Clinically Node-Negative Following Neo-Adjuvant Chemotherapy: A Literature Review. Curr. Oncol. 2023, 30, 8703-8719. https://doi.org/10.3390/curroncol30100630
Ferrarazzo G, Nieri A, Firpo E, Rattaro A, Mignone A, Guasone F, Manzara A, Perniciaro G, Spinaci S. The Role of Sentinel Lymph Node Biopsy in Breast Cancer Patients Who Become Clinically Node-Negative Following Neo-Adjuvant Chemotherapy: A Literature Review. Current Oncology. 2023; 30(10):8703-8719. https://doi.org/10.3390/curroncol30100630
Chicago/Turabian StyleFerrarazzo, Giulia, Alberto Nieri, Emma Firpo, Andrea Rattaro, Alessandro Mignone, Flavio Guasone, Augusto Manzara, Giuseppe Perniciaro, and Stefano Spinaci. 2023. "The Role of Sentinel Lymph Node Biopsy in Breast Cancer Patients Who Become Clinically Node-Negative Following Neo-Adjuvant Chemotherapy: A Literature Review" Current Oncology 30, no. 10: 8703-8719. https://doi.org/10.3390/curroncol30100630
APA StyleFerrarazzo, G., Nieri, A., Firpo, E., Rattaro, A., Mignone, A., Guasone, F., Manzara, A., Perniciaro, G., & Spinaci, S. (2023). The Role of Sentinel Lymph Node Biopsy in Breast Cancer Patients Who Become Clinically Node-Negative Following Neo-Adjuvant Chemotherapy: A Literature Review. Current Oncology, 30(10), 8703-8719. https://doi.org/10.3390/curroncol30100630