The Combined Therapy of Cabozantinib, Crizotinib, and Osimertinib in a Lung Cancer Patient with Acquired MET Amplification and Resistance Mutations
Abstract
:1. Introduction
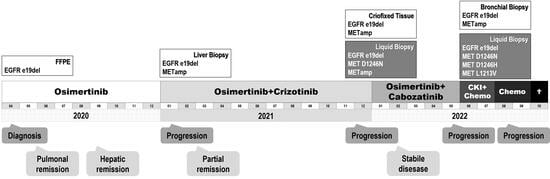
2. Case Presentation
3. Discussion
3.1. Applied Molecular Diagnostics
3.2. MET Amplification as a Resistance Driver
3.3. TP53 and Genomic Instability and TMB
3.4. Tumor Heterogeneity and Hybrid Capture
3.5. Possible Remaining Treatment Options after Cabozantinib
3.6. Patient Survival Benefits and Economic Considerations of Advanced Molecular Diagnostics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreated egfr-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Jóri, B.; Schatz, S.; Kaller, L.; Kah, B.; Roeper, J.; Ramdani, H.O.; Diehl, L.; Hoffknecht, P.; Grohé, C.; Griesinger, F. Comparison of resistance spectra after first and second line osimertinib treatment detected by liquid biopsy. Cancers 2021, 13, 2861. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in egfr-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Turke, A.B.; Zejnullahu, K.; Wu, Y.L.; Song, Y.; Dias-Santagata, D.; Lifshits, E.; Toschi, L.; Rogers, A.; Mok, T.; Sequist, L.; et al. Preexistence and clonal selection of met amplification in egfr mutant nsclc. Cancer Cell 2010, 17, 77–88. [Google Scholar] [CrossRef]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.-H.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S.; et al. Antitumor activity of crizotinib in lung cancers harboring a met exon 14 alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Lu, P.; Yu, Z.; Xu, C.; Zhuang, W.; Song, Z. Crizotinib with or without an egfr-tki in treating egfr-mutant nsclc patients with acquired met amplification after failure of egfr-tki therapy: A multicenter retrospective study. J. Transl. Med. 2019, 17, 52. [Google Scholar] [CrossRef]
- Gautschi, O.; Menon, R.; Bertrand, M.; Murer, C.; Diebold, J. Capmatinib and osimertinib combination therapy for egfr-mutant lung adenocarcinoma. J. Thorac. Oncol. 2020, 15, e13–e15. [Google Scholar] [CrossRef]
- Gautschi, O.; Diebold, J. Intracranial activity of osimertinib plus capmatinib in a patient with egfr and met-driven lung cancer: Case report. JTO Clin. Res. Rep. 2021, 2, 100162. [Google Scholar] [CrossRef]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular mechanisms of resistance to first- and second-generation alk inhibitors in alk-rearranged lung cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef]
- Tsai, J.M.; Hata, A.N.; Lennerz, J.K. “Met d1228n and d1246n are the same resistance mutation in met exon 14 skipping. Oncologist 2021, 26, e2297–e2301. [Google Scholar] [CrossRef]
- Pruis, M.A.; Geurts-Giele, W.R.R.; von der, T.J.H.; Meijssen, I.C.; Dinjens, W.N.M.; Aerts, J.; Dingemans, A.M.C.; Lolkema, M.P.; Paats, M.S.; Dubbink, H.J. Highly accurate DNA-based detection and treatment results of met exon 14 skipping mutations in lung cancer. Lung Cancer 2020, 140, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Heist, R.S.; Sequist, L.V.; Borger, D.; Gainor, J.F.; Arellano, R.S.; Le, L.P.; Dias-Santagata, D.; Clark, J.W.; Engelman, J.A.; Shaw, A.T.; et al. Acquired resistance to crizotinib in nsclc with met exon 14 skipping. J. Thorac. Oncol. 2016, 11, 1242–1245. [Google Scholar] [CrossRef]
- Lu, X.; Peled, N.; Greer, J.; Wu, W.; Choi, P.; Berger, A.H.; Wong, S.; Jen, K.Y.; Seo, Y.; Hann, B.; et al. Met exon 14 mutation encodes an actionable therapeutic target in lung adenocarcinoma. Cancer Res. 2017, 77, 4498–4505. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Wang, J.; Xu, P.; Zheng, Y.; Bai, L.; Sun, X.; Li, Z.; Gan, R.; Li, H.; Ke, Z.; et al. A rapid and durable response to cabozantinib in an osimertinib-resistant lung cancer patient with met d1228n mutation: A case report. Ann. Transl. Med. 2021, 9, 1354. [Google Scholar] [CrossRef] [PubMed]
- Pruis, M.A.; Paats, M.S.; Geurts, W.R.R.; Dubbink, H.J.; Dingemans, A.C. Overcoming acquired resistance mutation met d1228n to crizotinib with cabozantinib in nsclc with met exon 14 skipping mutation. JCO Precis. Oncol. 2021, 5, 849–853. [Google Scholar] [CrossRef] [PubMed]
- ClinVar National Center for Biotechnology Information. Nm_000546.6(tp53):C.559+1g>a. ClinVar. 2023. Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000428908.30 (accessed on 26 September 2023.).
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Schatz, S.; Falk, M.; Jóri, B.; Ramdani, H.O.; Schmidt, S.; Willing, E.M.; Menon, R.; Groen, H.J.M.; Diehl, L.; Kröger, M.; et al. “Integration of tumor mutation burden and pd-l1 testing in routine laboratory diagnostics in non-small cell lung cancer. Cancers 2020, 12, 1685. [Google Scholar] [CrossRef]
- Camidge, D.R.; Ou, S.-H.I.; Shapiro, G.; Otterson, G.A.; Villaruz, L.C.; Villalona-Calero, M.A.; Iafrate, A.J.; Varella-Garcia, M.; Dacic, S.; Cardarella, S.; et al. Efficacy and safety of crizotinib in patients with advanced c-met-amplified non-small cell lung cancer (nsclc). J. Clin. Oncol. 2014, 32, 8001. [Google Scholar] [CrossRef]
- Müller, J.N.; Falk, M.; Talwar, J.; Neemann, N.; Mariotti, E.; Bertrand, M.; Zacherle, T.; Lakis, S.; Menon, R.; Gloeckner, C.; et al. Concordance between comprehensive cancer genome profiling in plasma and tumor specimens. J. Thorac. Oncol. 2017, 12, 1503–1511. [Google Scholar] [CrossRef]
- Li, A.; Yang, J.J.; Zhang, X.C.; Zhang, Z.; Su, J.; Gou, L.Y.; Bai, Y.; Zhou, Q.; Yang, Z.; Han-Zhang, H.; et al. Acquired met y1248h and d1246n mutations mediate resistance to met inhibitors in non-small cell lung cancer. Clin. Cancer Res. 2017, 23, 4929–4937. [Google Scholar] [CrossRef]
- Fujino, T.; Kobayashi, Y.; Suda, K.; Koga, T.; Nishino, M.; Ohara, S.; Chiba, M.; Shimoji, M.; Tomizawa, K.; Takemoto, T.; et al. Sensitivity and resistance of met exon 14 mutations in lung cancer to eight met tyrosine kinase inhibitors in vitro. J. Thorac. Oncol. 2019, 14, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Riedel, R.; Heydt, C.; Scheel, A.H.; Tumbrink, H.L.; Brägelmann, J.; Castiglione, R.; Nogova, L.; Abdulla, D.S.Y.; Michels, S.Y.F.; Scheffler, M.; et al. Acquired resistance to met inhibition in met driven nsclc. J. Clin. Oncol. 2019, 37, 9030. [Google Scholar] [CrossRef]
- Riedel, R.; Fassunke, J.; Tumbrink, H.L.; Scheel, A.H.; Heydt, C.; Hieggelke, L.; Scheffler, M.; Heimsoeth, A.; Nogova, L.; Michels, S.; et al. Resistance to met inhibition in met-dependent nsclc and therapeutic activity after switching from type i to type ii met inhibitors. Eur. J. Cancer 2023, 179, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Roper, N.; Brown, A.L.; Wei, J.S.; Pack, S.; Trindade, C.; Kim, C.; Restifo, O.; Gao, S.; Sindiri, S.; Mehrabadi, F.; et al. Clonal evolution and heterogeneity of osimertinib acquired resistance mechanisms in egfr mutant lung cancer. Cell Rep. Med. 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Schmid, S.; Li, J.J.N.; Leighl, N.B. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer 2020, 147, 123–129. [Google Scholar] [CrossRef]
- Camidge, D.R.; Otterson, G.A.; Clark, J.W.; Ou, S.-H.I.; Weiss, J.; Ades, S.; Conte, U.; Tang, Y.; Wang, S.C.-E.; Murphy, D.; et al. Crizotinib in patients (pts) with met-amplified non-small cell lung cancer (nsclc): Updated safety and efficacy findings from a phase 1 trial. J. Clin. Oncol. 2018, 36, 9062. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in met exon 14–mutated or met-amplified non–small-cell lung cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 keynote-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Roeper, J.; Falk, M.; Chalaris-Rißmann, A.; Lueers, A.C.; Ramdani, H.; Wedeken, K.; Stropiep, U.; Diehl, L.; Tiemann, M.; Heukamp, L.C.; et al. Tp53 co-mutations in egfr mutated patients in nsclc stage iv: A strong predictive factor of orr, pfs and os in egfr mt+ nsclc. Oncotarget 2020, 11, 250–264. [Google Scholar] [CrossRef]
- Joshi, R.S.; Boichard, A.; Adashek, J.J.; Kurzrock, R. High tumor amplification burden is associated with tp53 mutations in the pan-cancer setting. Cancer Biol. Ther. 2022, 23, 1–6. [Google Scholar] [CrossRef]
- Wen, X.M.; Xu, Z.J.; Jin, Y.; Xia, P.H.; Ma, J.C.; Qian, W.; Lin, J.; Qian, J. Association analyses of tp53 mutation with prognosis, tumor mutational burden, and immunological features in acute myeloid leukemia. Front. Immunol. 2021, 12, 717527. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, K.; He, X.; Zhu, H.; Song, J.; Chen, S.; Cai, S.; Zhao, Y.; Wang, L. Lrp1b or tp53 mutations are associated with higher tumor mutational burden and worse survival in hepatocellular carcinoma. J. Cancer 2021, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, L.; Xie, X.; Qin, Y.; Xie, Z.; Ouyang, M.; Zhou, C. Prognostic biomarker tp53 mutations for immune checkpoint blockade therapy and its association with tumor microenvironment of lung adenocarcinoma. Front. Mol. Biosci. 2020, 7, 602328. [Google Scholar] [CrossRef]
- Assoun, S.; Theou-Anton, N.; Nguenang, M.; Cazes, A.; Danel, C.; Abbar, B.; Pluvy, J.; Gounant, V.; Khalil, A.; Namour, C.; et al. Association of tp53 mutations with response and longer survival under immune checkpoint inhibitors in advanced non-small-cell lung cancer. Lung Cancer 2019, 132, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Leshchiner, I.; Elagina, L.; Goyal, L.; Levovitz, C.; Siravegna, G.; Livitz, D.; Rhrissorrakrai, K.; Martin, E.E.; Van Seventer, E.E.; et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019, 25, 1415–1421. [Google Scholar] [CrossRef]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell, E.; Anesi, C.; Silvestris, N.; et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 95. [Google Scholar] [CrossRef]
- Smit, E.F.; Dooms, C.; Raskin, J.; Nadal, E.; Tho, L.M.; Le, X.; Mazieres, J.; Hin, H.S.; Morise, M.; Zhu, V.W.; et al. Insight 2: A phase ii study of tepotinib plus osimertinib in met-amplified nsclc and first-line osimertinib resistance. Future Oncol. 2022, 18, 1039–1054. [Google Scholar] [CrossRef]
- Shu, C.A.; KGoto; Ohe, Y.; Besse, B.; Lee, S.-H.; Wang, Y.; Griesinger, F.; Yang, J.C.-H.; Felip, E.; Sanborn, R.E.; et al. Amivantamab and lazertinib in patients with egfr-mutant non–small cell lung (nsclc) after progression on osimertinib and platinum-based chemotherapy: Updated results from chrysalis-2. J. Clin. Oncol. 2022, 40, 9006. [Google Scholar] [CrossRef]
- Bahcall, M.; Paweletz, C.P.; Kuang, Y.; Taus, L.J.; Sim, T.; Kim, N.D.; Dholakia, K.H.; Lau, C.J.; Gokhale, P.C.; Chopade, P.R.; et al. Combination of type i and type ii met tyrosine kinase inhibitors as therapeutic approach to prevent resistance. Mol. Cancer Ther. 2022, 21, 322–335. [Google Scholar] [CrossRef]
- Zou, D.; Ye, W.; Hess, L.M.; Bhandari, N.R.; Ale-Ali, A.; Foster, J.; Quon, P.; Harris, M. Diagnostic value and cost-effectiveness of next-generation sequencing-based testing for treatment of patients with advanced/metastatic non-squamous non-small-cell lung cancer in the united states. J. Mol. Diagn. 2022, 24, 901–914. [Google Scholar] [CrossRef]



| Date | April 2020 | January 2021 | December 2021 | January 2022 | May 2022 | May 2022 | |||
|---|---|---|---|---|---|---|---|---|---|
| Assay | NEO- Plus | NEO- Select | NEO- Liquid | HS2- Lung | HS2- Lung | HS- Liquid | |||
| Sample Type | FFPE | FFPE | Eff. | FFPE | FFPE | Cf | |||
| Point Mutations detected with HC-NGS | |||||||||
| Gen | Transcript. | DNA-change | Protein change | Variant Allelic Frequency | |||||
| EGFR | NM_005228.4 | c.2236_2250del | p.E746_A750del | 17% | 56% | 7% | 27% | 39% | 60% |
| TP53 | NM_001126112.2 | c.559 + 1G > A | p.? | 12% | 61% | 7% | 15% | 31% | 41% |
| MET | NM_00112750.3 (NM_000245.4) | c.3736G > A (c.3682G > A) | p.D1246N (p.D1228N) | - | - | 0.1% | - | - | 0.7% |
| MET | NM_00112750.3 (NM_000245.4) | c.3736G > C (c.3682G > C) | p.D1246H (p.D1228H) | - | - | - | - | - | 0.4% |
| MET | NM_00112750.3 (NM_000245.4) | c.3637C > G (c.3583C > G) | p.L1213V (p.L1195V) | - | - | - | - | - | 0.5% |
| Amplifications detected with HC-NGS | |||||||||
| Gen | Transcript | Level 1 | |||||||
| EGFR | NM_005228.4 | - | + | - | + | + | n.a. | ||
| MET | NM_00112750.3 | - | ++ | ++ | ++ | ++ | n.a. | ||
| Additional alterations detected with HC-NGS | |||||||||
| Tumor Mutational Burden (Muts/mB) | 17.42 | - | - | - | - | - | |||
| Microsatellite Instability | MSI-L | - | - | - | - | - | |||
| TP53, NRAS, BRCA2, and ARAF | - | DEL | - | - | - | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóri, B.; Vössing, C.; Pirngruber, J.; Willing, E.M.; Arndt, K.; Falk, M.; Tiemann, M.; Heukamp, L.C.; Hoffknecht, P. The Combined Therapy of Cabozantinib, Crizotinib, and Osimertinib in a Lung Cancer Patient with Acquired MET Amplification and Resistance Mutations. Curr. Oncol. 2023, 30, 8805-8814. https://doi.org/10.3390/curroncol30100635
Jóri B, Vössing C, Pirngruber J, Willing EM, Arndt K, Falk M, Tiemann M, Heukamp LC, Hoffknecht P. The Combined Therapy of Cabozantinib, Crizotinib, and Osimertinib in a Lung Cancer Patient with Acquired MET Amplification and Resistance Mutations. Current Oncology. 2023; 30(10):8805-8814. https://doi.org/10.3390/curroncol30100635
Chicago/Turabian StyleJóri, Balázs, Christine Vössing, Judith Pirngruber, Eva Maria Willing, Kathrin Arndt, Markus Falk, Markus Tiemann, Lukas C. Heukamp, and Petra Hoffknecht. 2023. "The Combined Therapy of Cabozantinib, Crizotinib, and Osimertinib in a Lung Cancer Patient with Acquired MET Amplification and Resistance Mutations" Current Oncology 30, no. 10: 8805-8814. https://doi.org/10.3390/curroncol30100635
APA StyleJóri, B., Vössing, C., Pirngruber, J., Willing, E. M., Arndt, K., Falk, M., Tiemann, M., Heukamp, L. C., & Hoffknecht, P. (2023). The Combined Therapy of Cabozantinib, Crizotinib, and Osimertinib in a Lung Cancer Patient with Acquired MET Amplification and Resistance Mutations. Current Oncology, 30(10), 8805-8814. https://doi.org/10.3390/curroncol30100635