Robotic versus Open Surgery in Locally Advanced Non-Small Cell Lung Cancer: Evaluation of Surgical and Oncological Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Details
2.2. Data Analysis
3. Results
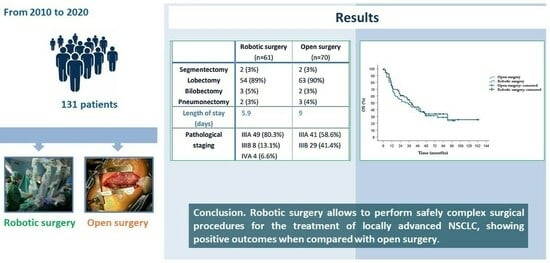
3.1. Clinical Characteristics of the Patients
3.2. Operative and Postoperative Results
3.3. Oncologic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, A.E. Surgical Management of Lung Cancer: History, Evolution, and Modern Advances. Curr. Oncol. Rep. 2018, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.S.; Reddy, R.M.; Gorrepati, M.L.; Mehendale, S.; Reed, M.F. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann. Thorac. Surg. 2017, 104, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.S.; Hartwig, M.G.; Vallières, E.; Abbas, A.E.; Cerfolio, R.J.; Dylewski, M.R.; Fabian, T.; Herrera, L.J.; Jett, K.G.; Lazzaro, R.S.; et al. Pulmonary Open, Robotic and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann. Surg. 2021, 277, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Mariolo, A.V.; Mohamed, S.; Sedda, G.; Maisonneuve, P.; Mazzella, A.; Lo Iacono, G.; Petrella, F.; Spaggiari, L. Long-Term Outcomes of Robotic-Assisted, Video-Assisted and Open Surgery in Non-Small Cell Lung Cancer: A Matched Analysis. J. Clin. Med. 2022, 11, 3363. [Google Scholar] [CrossRef] [PubMed]
- Zirafa, C.C.; Romano, G.; Sicolo, E.; Cariello, C.; Morganti, R.; Conoscenti, L.; Hung-Key, T.; Davini, F.; Melfi, F. Robotic Surgery for Non-Small Cell Lung Cancer Treatment in High-Risk Patients. J. Clin. Med. 2021, 10, 4408. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, L.; Tang, J.; Cheng, J.; Chen, N.; Zhou, J.; Liu, L. Video-assisted thoracoscopic surgery versus muscle-sparing thoracotomy for non-small cell lung cancer: A systematic review and meta-analysis. BMC Surg. 2019, 19, 144. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, D.; Fieira, E.; Delgado, M.; Mendez, L.; Fernandez, R.; de la Torre, M. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J. Thorac. Dis. 2014, 6, 641–648. [Google Scholar] [CrossRef]
- Yang, C.F.; Meyerhoff, R.R.; Mayne, N.R.; Singhapricha, T.; Toomey, C.B.; Speicher, P.J.; Hartwig, M.G.; Tong, B.C.; Onaitis, M.W.; Harpole, D.H., Jr.; et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2016, 49, 1615–1623. [Google Scholar] [CrossRef]
- Scheinerman, J.A.; Jiang, J.; Chang, S.H.; Geraci, T.C.; Cerfolio, R.J. Extended Robotic Pulmonary Resections. Front. Surg. 2021, 8, 597416. [Google Scholar] [CrossRef]
- Herrera, L.J.; Wherley, E.M.; Agyabeng-Dadzie, K.; Ramsuchit, B.; Johnston, M.A.; Escalon, J.C. 500 Consecutive Robotic Lobectomies for Non-Small Cell Lung Cancer: Perioperative and Oncologic Outcomes. Innovations 2021, 16, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rusch, V.W.; Jones, D.R.; et al. Long-term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann. Surg. 2017, 265, 431–437. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; D’Souza, D.M.; Richardson, M.; Abdel-Rasoul, M.; Moffatt-Bruce, S.D.; Merritt, R.E. Long-Term Oncologic Outcomes After Robotic Lobectomy for Early-stage Non-Small-cell Lung Cancer Versus Video-assisted Thoracoscopic and Open Thoracotomy Approach. Clin. Lung Cancer 2020, 21, 214–224.e2. [Google Scholar] [CrossRef] [PubMed]
- NCCN Non-Small-Cell-Lung-Cancer Guidelines. Version 1.2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (accessed on 1 October 2022).
- Tam, K.; Daly, M.; Kelly, K. Treatment of Locally Advanced Non-Small Cell Lung Cancer. Hematol. Oncol. Clin. N. Am. 2017, 31, 45–57. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.M.; Kauffmann-Guerrero, D.; Hoffmann, H.; Flentje, M. New developments in locally advanced non-small cell lung cancer. Eur. Respir. Rev. 2021, 30, 200227. [Google Scholar] [CrossRef] [PubMed]
- Bryan, D.S.; Donington, J.S. The Role of Surgery in Management of Locally Advanced Non-Small Cell Lung Cancer. Curr. Treat. Options Oncol. 2019, 20, 27. [Google Scholar] [CrossRef]
- Mason, D.P. The role of surgery for locally advanced non-small cell lung cancer. Cleve. Clin. J. Med. 2012, 79 (Suppl. S1), eS38–eS41. [Google Scholar] [CrossRef]
- Speicher, P.J.; Englum, B.R.; Ganapathi, A.M.; Onaitis, M.W.; D’Amico, T.A.; Berry, M.F. Outcomes after treatment of 17,378 patients with locally advanced (T3N0-2) non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2015, 47, 636–641. [Google Scholar] [CrossRef]
- Hennon, M.W.; Demmy, T.L. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann. Cardiothorac. Surg. 2012, 1, 37–42. [Google Scholar]
- Gonfiotti, A.; Bongiolatti, S.; Bertolaccini, L.; Viggiano, D.; Solli, P.; Droghetti, A.; Bertani, A.; Crisci, R.; Voltolini, L. Italian VATS Group. Thoracoscopic lobectomy for locally advanced-stage non-small cell lung cancer is a feasible and safe approach: Analysis from multi-institutional national database. J. Vis. Surg. 2017, 3, 160. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, L.; Wang, Y.; Lv, W.; Hu, J. Video assisted thoracic surgery vs. thoracotomy for locally advanced lung squamous cell carcinoma after neoadjuvant chemotherapy. J. Cardiothorac. Surg. 2018, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Medbery, R.L.; Gillespie, T.W.; Liu, Y.; Nickleach, D.C.; Lipscomb, J.; Sancheti, M.S.; Pickens, A.; Force, S.D.; Fernandez, F.G. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J. Thorac. Oncol. 2016, 11, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; Louie, B.E.; Cerfolio, R.J.; Park, B.J.; Vallières, E.; Aye, R.W.; Abdel-Razek, A.; Bryant, A.; Farivar, A.S. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann. Thorac. Surg. 2014, 97, 1901–1906; discussion 1906–1907. [Google Scholar] [CrossRef]
- Gallina, F.T.; Melis, E.; Forcella, D.; Mercadante, E.; Marinelli, D.; Ceddia, S.; Cappuzzo, F.; Vari, S.; Cecere, F.L.; Caterino, M.; et al. Nodal Upstaging Evaluation After Robotic-Assisted Lobectomy for Early-Stage Non-small Cell Lung Cancer Compared to Video-Assisted Thoracic Surgery and Thoracotomy: A Retro-spective Single Center Analysis. Front. Surg. 2021, 8, 666158. [Google Scholar] [CrossRef]
- Patané, A.K. Minimal invasive surgery in locally advanced N2 non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 519–528. [Google Scholar] [CrossRef]
- Park, B.J.; Yang, H.X.; Woo, K.M.; Sima, C.S. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J. Thorac. Dis. 2016, 8 (Suppl. S4), S406–S413. [Google Scholar] [CrossRef]
- Veronesi, G.; Park, B.; Cerfolio, R.; Dylewski, M.; Toker, A.; Fontaine, J.P.; Hanna, W.C.; Morenghi, E.; Novellis, P.; Velez-Cubian, F.O.; et al. Robotic resection of Stage III lung cancer: An international retrospective study. Eur. J. Cardiothorac. Surg. 2018, 54, 912–919. [Google Scholar] [CrossRef]
- Patel, Y.S.; Baste, J.M.; Shargall, Y.; Waddell, T.K.; Yasufuku, K.; Machuca, T.N.; Xie, F.; Thabane, L.; Hanna, W.C. Robotic Lobectomy is Cost-Effective and Provides Comparable Health Utility Scores to Video-Assisted Lobectomy: Early Results of the RAVAL Trial. Ann. Surg, 2023; Online ahead of print. [Google Scholar]
- Nguyen, D.M.; Sarkaria, I.S.; Song, C.; Reddy, R.M.; Villamizar, N.; Herrera, L.J.; Shi, L.; Liu, E.; Rice, D.; Oh, D.S. Clinical and economic comparative effectiveness of robotic-assisted, video-assisted thoracoscopic, and open lobectomy. J. Thorac. Dis. 2020, 12, 296–306. [Google Scholar] [CrossRef]
- Shah, P.C.; de Groot, A.; Cerfolio, R.; Huang, W.C.; Huang, K.; Song, C.; Li, Y.; Kreaden, U.; Oh, D.S. Impact of type of minimally invasive approach on open conversions across ten common procedures in different specialties. Surg. Endosc. 2022, 36, 6067–6075. [Google Scholar] [CrossRef]
- Herb, J.N.; Kindell, D.G.; Strassle, P.D.; Stitzenberg, K.B.; Haithcock, B.E.; Mody, G.N.; Long, J.M. Trends and Outcomes in Minimally Invasive Surgery for Locally Advanced Non-Small-Cell Lung Cancer with N2 Disease. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, Y.; Huang, J.; Li, J.; Jiang, L.; Lin, H.; Lu, P.; Luo, Q. Comparison of robotic-assisted lobectomy with video-assisted thoracic surgery for stage IIB-IIIA non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Shahin, G.M.M.; Vos, P.W.K.; Hutteman, M.; Stigt, J.A.; Braun, J. Robot-assisted thoracic surgery for stages IIB-IVA non-small cell lung cancer: Retrospective study of feasibility and outcome. J. Robot Surg. 2023, 17, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Hennon, M.; Sahai, R.K.; Yendamuri, S.; Tan, W.; Demmy, T.L.; Nwogu, C. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann. Surg. Oncol. 2011, 18, 3732–3736. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, T.; Zhang, Q.; Li, L.; Xu, C. A systematic review and meta-analysis of neoadjuvant chemoimmunotherapy in stage III non-small cell lung cancer. BMC Pulm. Med. 2022, 22, 490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Y.; Gao, Y.; Sugimura, H.; Minervini, F.; Uchino, J.; Halmos, B.; Yendamuri, S.; Velotta, J.B.; Li, M. Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2022, 11, 277–294. [Google Scholar] [CrossRef]
- Cao, C.; Guo, A.; Chen, C.; Chakos, A.; Bott, M.; Yang, C.J.; Zielinski, R.; Melfi, F. Systematic Review of Neoadjuvant Immunotherapy for Patients with Non-Small Cell Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 850–857. [Google Scholar] [CrossRef]



| Robotic Surgery (n = 61) | Open Surgery (n = 70) | p-Value | |
|---|---|---|---|
| Sex | 0.196 | ||
| Male | 38 (62%) | 51 (73%) | |
| Female | 23 (38%) | 19 (27%) | |
| Age (years) | 67.3 | 69.4 | 0.134 |
| Smokers | 0.635 | ||
| Current | 16 (26%) | 17 (24%) | |
| Former | 38 (62%) | 41 (59%) | |
| FEV1 (litres) | 2.51 (0.77) | 2.4 (0.60) | 0.613 |
| BMI | 26.6 (4.67) | 25.7 (3.65) | 0.210 |
| ASA score | 0.645 | ||
| 2 | 26 (43%) | 26 (37%) | |
| 3 | 35 (57%) | 44 (63%) | |
| CCI score | 0.532 | ||
| 0–4 | 46 (75%) | 57 (81%) | |
| ≥5 | 15 (25%) | 13 (19%) | |
| T parameter | 0.005 | ||
| T1 + T2 | 32 (53.5) | 20 (28.6%) | |
| T3 +T4 | 29 (47.5) | 50 (71.4) |
| Robotic Approach (n = 61) | Open Approach (n = 70) | |
|---|---|---|
| Surgical procedure | 2 segmentectomies | 2 segmentectomies |
| 54 lobectomies | 63 lobectomies | |
| 11 RUL | 22 RUL | |
| 1 ML | 4 ML | |
| 18 RLL | 10 RLL | |
| 11 LUL | 11 LUL | |
| 13 LLL | 16 LLL | |
| 3 bilobectomies | 2 bilobectomies | |
| 2 pneumonectomies | 3 pneumonectomies | |
| Histology | Adenocarcinoma 46 (75.4%) | Adenocarcinoma 45 (64.2%) |
| Squamous cell carcinoma 9 (14.7%) | Squamous cell carcinoma 21 (30%) | |
| Others 6 (9.8%) | Others 4 (5.8%) | |
| Lymph nodes removed | 18.9 (1–42) | 19.4 (1–47) |
| N stations | 5.1 (1–8) | 5.1 (1–7) |
| N2 stations | 3.3 (1–5) | 3.2 (0–6) |
| pN | N0 5 (8.2%) | N0 4 (5.7%) |
| N1 16 (26.2%) | N1 17 (24.3%) | |
| N2 40 (65.6%) | N2 49 (70%) | |
| Pathological staging | IIIA 49 (80.3%) | IIIA 41 (58.6%) IIIB 29 (41.4%) |
| IIIB 8 (13.1%) | ||
| IVA 4 (6.6%) |
| Factor | HR | 95% CI–Lower | 95% CI–Upper | p-Value |
|---|---|---|---|---|
| Robotic surgery: (0) no, (1) yes | 0.891 | 0.574 | 1.385 | 0.609 |
| Age | 1.025 | 0.998 | 1.054 | 0.073 |
| Gender: (0) M, (1) F | 0.744 | 0.461 | 1.200 | 0.225 |
| BMI | 0.975 | 0.925 | 1.027 | 0.341 |
| Smoker (0) no, (1) former, (2) yes | 1.048 | 0.744 | 1.477 | 0.787 |
| ASA score | 1.570 | 1.004 | 2.455 | 0.048 |
| Charlson comorbidity index | 1.147 | 0.989 | 1.331 | 0.070 |
| FEV1 | 0.945 | 0.691 | 1.292 | 0.723 |
| Lobectomy | 0.799 | 0.292 | 2.184 | 0.661 |
| Adenocarcinoma | 0.912 | 0.569 | 1.463 | 0.703 |
| Squamous carcinoma: (0) no, (1) yes | 1.200 | 0.717 | 2.008 | 0.488 |
| N (0–2) | 1.135 | 0.782 | 1.649 | 0.504 |
| Stage (3–4) | 0.807 | 0.294 | 2.215 | 0.677 |
| T parameter: (0) T2 + T3, (1) T3 + T4 | 1.432 | 0.902 | 2.275 | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirafa, C.C.; Romano, G.; Sicolo, E.; Bagalà, E.; Manfredini, B.; Alì, G.; Castaldi, A.; Morganti, R.; Davini, F.; Fontanini, G.; et al. Robotic versus Open Surgery in Locally Advanced Non-Small Cell Lung Cancer: Evaluation of Surgical and Oncological Outcomes. Curr. Oncol. 2023, 30, 9104-9115. https://doi.org/10.3390/curroncol30100658
Zirafa CC, Romano G, Sicolo E, Bagalà E, Manfredini B, Alì G, Castaldi A, Morganti R, Davini F, Fontanini G, et al. Robotic versus Open Surgery in Locally Advanced Non-Small Cell Lung Cancer: Evaluation of Surgical and Oncological Outcomes. Current Oncology. 2023; 30(10):9104-9115. https://doi.org/10.3390/curroncol30100658
Chicago/Turabian StyleZirafa, Carmelina C., Gaetano Romano, Elisa Sicolo, Elena Bagalà, Beatrice Manfredini, Greta Alì, Andrea Castaldi, Riccardo Morganti, Federico Davini, Gabriella Fontanini, and et al. 2023. "Robotic versus Open Surgery in Locally Advanced Non-Small Cell Lung Cancer: Evaluation of Surgical and Oncological Outcomes" Current Oncology 30, no. 10: 9104-9115. https://doi.org/10.3390/curroncol30100658
APA StyleZirafa, C. C., Romano, G., Sicolo, E., Bagalà, E., Manfredini, B., Alì, G., Castaldi, A., Morganti, R., Davini, F., Fontanini, G., & Melfi, F. (2023). Robotic versus Open Surgery in Locally Advanced Non-Small Cell Lung Cancer: Evaluation of Surgical and Oncological Outcomes. Current Oncology, 30(10), 9104-9115. https://doi.org/10.3390/curroncol30100658