CO2 and CH4 Adsorption Behavior of Biomass-Based Activated Carbons
Abstract
:1. Introduction
2. Materials
2.1. Sample Preparation
2.1.1. Physical Activation
2.1.2. Chemical Activation
2.2. Samples Properties
3. Experimental Methodology
3.1. High Pressure Manometric Adsorption Setup
3.2. Determination of Excess Adsorption
3.3. Parametrization of Excess Adsorption Isotherms
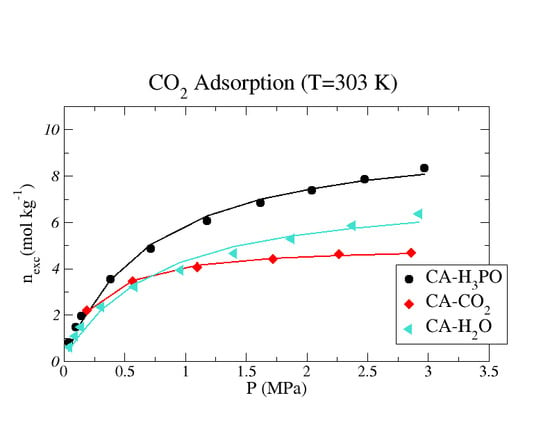
4. Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Comission. Paris Agreement. Available online: https://ec.europa.eu/clima/policies/international/negotiations/parisen (accessed on 12 November 2018).
- European Comission. Renewable Energy. Moving towards a low carbon economy. Available online: https://ec.europa.eu/energy/en/topics/renewable-energy (accessed on 12 November 2018).
- Starr, K.; Villalba, G.; Gabarrell, X. Upgraded biogas from municipal solid waste for natural gas substitution and CO2 reduction—A case study of Austria, Italy, and Spain. Waste Manag. 2015, 38, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- EBA European Biogas Association. EBA Statictical Report 2017 Published Soon. Available online: http://european-biogas.eu/2017/12/14/eba-statistical-report-2017-published-soon/ (accessed on 12 November 2018).
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ling, J.; Xiao, P.; He, Y.; Zhao, Q.; Chu, Z.; Liu, Y.; Li, Z.; Webley, P.A. Simultaneous biogas purification and CO2 capture by vacuum swing adsorption using zeolite NaUSY. Chem. Eng. J. 2018, 334, 2593–2602. [Google Scholar] [CrossRef]
- Gong, H.; Lee, S.S.; Bae, T.H. Mixed-matrix membranes containing inorganically surface-modified 5A zeolite for enhanced CO2/CH4 separation. Microporous Mesoporous Mater. 2017, 237, 82–89. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Tezel, F.H. Cation exchange modification of clinoptilolite—Screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide. Microporous Mesoporous Mater. 2018, 262, 235–250. [Google Scholar] [CrossRef]
- Son, S.J.; Choi, J.S.; Choo, K.Y.; Song, S.D.; Vijayalakshmi, S.; Kim, T.H. Development of carbon dioxide adsorbents using carbon materials prepared from coconut shell. Korean J. Chem. Eng. 2005, 22, 291–297. [Google Scholar] [CrossRef]
- Rocha, L.A.; Andreassen, K.A.; Grande, C.A. Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure. Chem. Eng. Sci. 2017, 164, 148–157. [Google Scholar] [CrossRef]
- Arya, A.; Divekar, S.; Rawat, R.; Gupta, P.; Garg, M.O.; Dasgupta, S.; Nanoti, A.; Singh, R.; Xiao, P.; Webley, P.A. Upgrading biogas at low pressure by vacuum swing adsorption. Ind. Eng. Chem. Res. 2015, 54, 404–413. [Google Scholar] [CrossRef]
- Samarasinghe, S.A.; Chuah, C.Y.; Yang, Y.; Bae, T.H. Tailoring CO2/CH4 separation properties of mixed-matrix membranes via combined use of two- and three-dimensional metal-organic frameworks. J. Membr. Sci. 2018, 557, 30–37. [Google Scholar] [CrossRef]
- Zacharia, R.; Gomez, L.F.; Chahine, R.; Cossement, D.; Benard, P. Thermodynamics and kinetics of CH4/CO2 binary mixture separation by metal-organic frameworks from isotope exchange and adsorption break-through. Microporous Mesoporous Mater. 2018, 263, 165–172. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Jia, C.; Wang, Y.; Zhai, L.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Yao, K.X.; Chen, Y.; Lu, Y.; Zhao, Y.; Ding, Y. Ultramicroporous carbon with extremely narrow pore distribution and very high nitrogen doping for efficient methane mixture gases upgrading. Carbon 2017, 122, 258–265. [Google Scholar] [CrossRef]
- Koonaphapdeelert, S.; Moran, J.; Aggarangsi, P.; Bunkham, A. Low pressure biomethane gas adsorption by activated carbon. Energy Sustain. Dev. 2018, 43, 196–202. [Google Scholar] [CrossRef]
- Saha, D.; Nelson, K.; Chen, J.; Lu, Y.; Ozcan, S. Adsorption of CO2 ,CH4, and N2 in Micro-Mesoporous Nanographene: A Comparative Study. J. Chem. Eng. Data 2015, 60, 2636–2645. [Google Scholar] [CrossRef]
- Vilella, P.C.; Lira, J.A.; Azevedo, D.C.; Bastos-Neto, M.; Stefanutti, R. Preparation of biomass-based activated carbons and their evaluation for biogas upgrading purposes. Ind. Crops Prod. 2017, 109, 134–140. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Yadavalli, G.; Lei, H.; Wei, Y.; Zhu, L.; Zhang, X.; Liu, Y.; Yan, D. Carbon dioxide capture using ammonium sulfate surface modified activated biomass carbon. Biomass Bioenergy 2017, 98, 53–60. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Fuente, E. Influence of the pyrolysis step and the tanning process on KOH-activated carbons from biocollagenic wastes. Prospects as adsorbent for CO2 capture. J. Anal. Appl. Pyrolysis 2014, 110, 194–204. [Google Scholar] [CrossRef]
- Bagheri, N.; Abedi, J. Adsorption of methane on corn cobs based activated carbon. Chem. Eng. Res. Des. 2011, 89, 2038–2043. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2011, 89, 657–664. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.j.; Hong, Y.z.; Cao, F.; Li, L.; Li, J.b. Preparation of high-surface-area activated carbon from coconut shell fibers. Carbon 2010, 48, 3005. [Google Scholar] [CrossRef]
- Iley, M.; Marsh, H.; Rodriguez-Reinoso, F. The Adsorptive Properties of Carbonised Olive Stones. Carbon 1973, 11, 633–636. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, G.; Hou, H.; Ge, P.; Cao, X.; Ji, X. Sulfur-doped carbon employing biomass-activated carbon as a carrier with enhanced sodium storage behavior. J. Mater. Chem. A 2017, 5, 24353–24360. [Google Scholar] [CrossRef]
- Yakout, S.M.; Sharaf El-Deen, G. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular Bamboo-Derived Activated Carbon for High CO2 Adsorption: The Dominant Role of Narrow Micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gutiérrez, N.; García, S.; Gil, M.V.; Rubiera, F.; Pevida, C. Towards bio-upgrading of biogas: Biomass waste-based adsorbents. Energy Procedia 2014, 63, 6527–6533. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sust. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Gil, M.V.; Martínez, M.; García, S.; Rubiera, F.; Pis, J.J.; Pevida, C. Response surface methodology as an efficient tool for optimizing carbon adsorbents for CO2 capture. Fuel Process. Technol. 2013, 106, 55–61. [Google Scholar] [CrossRef]
- Djeridi, W.; Ben Mansour, N.; Ouederni, A.; Llewellyn, P.L.; El Mir, L. Influence of the raw material and nickel oxide on the CH4 capture capacity behaviors of microporous carbon. Int. J. Hydrogen Energy 2015, 40, 13690–13701. [Google Scholar] [CrossRef]
- Soudani, N.; Najar-Souissi, S.; Abderkader-Fernandez, V.; Ouederni, A. Effects of nitrogen plasma treatment on the surface characteristics of olive stone-based activated carbon. Environ. Technol. 2017, 38, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, M.; Tsyntsarski, B.; Erto, A.; Budinova, T.; Petrova, B.; Petrov, N.; Lancia, A. Dynamic studies on carbon dioxide capture using lignocellulosic based activated carbons. Adsorption 2015, 21, 633–643. [Google Scholar] [CrossRef]
- Álvarez-Gutiérrez, N.; García, S.; Gil, M.V.; Rubiera, F.; Pevida, C. Dynamic Performance of Biomass- Based Carbons for CO2/CH4 Separation. Approximation to a Pressure Swing Adsorption Process for Biogas Upgrading. Energy Fuels 2016, 30, 5005–5015. [Google Scholar] [CrossRef]
- Erto, A.; Tsyntsarski, B.; Balsamo, M.; Budinova, T.; Lancia, A.; Petrova, B.; Petrov, N. Synthesis of Activated Carbons by Thermal Treatments of Agricultural Wastes for CO2 Capture from Flue Gas. Combust. Sci. Technol. 2016, 188, 581–593. [Google Scholar] [CrossRef]
- Moussa, M.; Bader, N.; Querejeta, N.; Durán, I.; Pevida, C.; Ouederni, A. Toward sustainable hydrogen storage and carbon dioxide capture in post-combustion conditions. J. Environ. Chem. Eng. 2017, 5, 1628–1637. [Google Scholar] [CrossRef] [Green Version]
- Balsamo, M.; Silvestre-Albero, A.; Silvestre-Albero, J.; Erto, A.; Rodríguez-Reinoso, F.; Lancia, A. Assessment of CO2 adsorption capacity on activated carbons by a combination of batch and dynamic tests. Langmuir 2014, 30, 5840–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghouma, I.; Jeguirim, M.; Sager, U.; Limousy, L.; Bennici, S.; Däuber, E.; Asbach, C.; Ligotski, R.; Schmidt, F.; Ouederni, A. The potential of activated carbon made of agro-industrial residues in NOx immissions abatement. Energies 2017, 10, 508. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Dorge, S.; Limousy, L.; Matei Ghimbeu, C.; Ouederni, A. Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. Comptes Rendus Chimie 2015, 18, 63–74. [Google Scholar] [CrossRef]
- Limousy, L.; Ghouma, I.; Ouederni, A.; Jeguirim, M. Amoxicillin removal from aqueous solution using activated carbon prepared by chemical activation of olive stone. Environ. Sci. Pollut. R. 2017, 24, 9993–10004. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; Álvarez-Murillo, A.; Al-Kassir, A.; Yusaf, T. Dependence of the microporosity of activated carbons on the lignocellulosic composition of the precursors. Energies 2017, 10, 542. [Google Scholar] [CrossRef]
- Song, T.; Liao, J.M.; Xiao, J.; Shen, L.H. Effect of micropore and mesopore structure on CO2 adsorption by activated carbons from biomass. Xinxing Tan Cailiao/New Carbon Mater. 2015, 30, 156–166. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.; Freitas, M.; Órfão, J. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Ortiz Cancino, O.P.; Peredo Mancilla, D.; Pozo, M.; Pérez, E.; Bessieres, D. Effect of Organic Matter and Thermal Maturity on Methane Adsorption Capacity on Shales from the Middle Magdalena Valley Basin in Colombia. Energy Fuels 2017, 31, 11698–11709. [Google Scholar] [CrossRef]
- Peredo-Mancilla, D.; Hort, C.; Jeguirim, M.; Ghimbeu, C.M.; Limousy, L.; Bessieres, D. Experimental Determination of the CH4 and CO2 Pure Gas Adsorption Isotherms on Different Activated Carbons. J. Chem. Eng. Data 2018. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology NIST, U.S. Secretary of Commerce. Isothermal Properties for Carbon Dioxide. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Name=carbon+dioxideUnits=SI (accessed on 12 November 2018).
- Gensterblum, Y.; Merkel, A.; Busch, A.; Krooss, B.M. High-pressure CH4 and CO2 sorption isotherms as a function of coal maturity and the influence of moisture. Int. J. Coal Geol. 2013, 118, 45–57. [Google Scholar] [CrossRef]
- Tóth, J. Uniform interpretation of gas/solid adsorption. Adv. Colloid Interface Sci. 1995, 55, 1–239. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A.; Quinn, D.F. Influence of pore size distribution on methane storage at relatively low pressure: Preparation of activated carbon with optimum pore size. Carbon 2002, 40, 989–1002. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Gadea-Ramos, E.; Kaneko, K.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. High-pressure methane storage in porous materials: Are carbon materials in the pole position? Chem. Mater. 2015, 27, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, P.; Wachowska, H.; Pietrzak, R. Active carbons prepared by chemical activation of plum stones and their application in removal of NO2. J. Hazard. Mater. 2010, 181, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wilcox, J. Molecular Simulation Studies of CO2 Adsorption by Carbon Model Compounds for Carbon Capture and Sequestration Applications. Environ. Sci. Technol. 2013, 47, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liu, C.; Zhou, Z.; Zhou, J.; Wang, G.; Zhuo, S.; Xue, Q.; Song, L.; Yan, Z. Oxygen-containing functional group-facilitated CO2 capture by carbide-derived carbons. Nanoscale Res. Lett. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vivo-Vilches, J.F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Faria, R.P.V.; Ribeiro, A.M.; Ferreira, A.F.P.; Rodrigues, A.E. Biogas upgrading by selective adsorption onto CO2 activated carbon from wood pellets. J. Environ. Chem. Eng. 2017, 5, 1386–1393. [Google Scholar] [CrossRef]
- Munusamy, K.; Somani, R.S.; Bajaj, H.C. Breakthrough adsorption studies of mixed gases on mango (Mangifera indica L.) seed shell derived activated carbon extrudes. J. Environ. Chem. Eng. 2015, 3, 2750–2759. [Google Scholar] [CrossRef]




| Sample | (m g) | (cm g) | (cm g) | (cm g) |
|---|---|---|---|---|
| AC-HPO | 1178 | 0.45 | 0.49 | 0.04 |
| AC-CO | 757 | 0.30 | 0.32 | 0.02 |
| AC-HO | 754 | 0.28 | 0.58 | 0.30 |
| Sample | CO (mmol g) | CO (mmol g) |
|---|---|---|
| AC-HPO | 3.43 | 0.72 |
| AC-CO | 1.06 | 0.38 |
| AC-HO | 1.25 | 0.39 |
| CH Adsorption | ||||
|---|---|---|---|---|
| Sample | Temperature (K) | (mol kg) | (MPa) | |
| AC-HPO | 303.15 | 6.518 | 0.932 | 0.042 |
| 323.15 | 6.369 | 1.182 | 0.037 | |
| AC-CO | 303.15 | 3.913 | 0.273 | 0.043 |
| 323.15 | 3.830 | 0.076 | 0.031 | |
| AC-HO | 303.15 | 5.417 | 0.714 | 0.067 |
| 323.15 | 5.301 | 1.011 | 0.056 | |
| CO Adsorption | ||||
|---|---|---|---|---|
| Sample | Temperature (K) | (mol kg) | (MPa) | |
| AC-HPO | 303.15 | 10.873 | 0.488 | 0.080 |
| 323.15 | 10.254 | 0.733 | 0.065 | |
| AC-CO | 303.15 | 5.878 | 0.181 | 0.059 |
| 323.15 | 5.191 | 0.273 | 0.020 | |
| AC-HO | 303.15 | 7.968 | 0.371 | 0.073 |
| 323.15 | 7.721 | 0.772 | 0.087 | |
| CO and CH Adsorption Capacity | |||||
|---|---|---|---|---|---|
| Sample | Precursor | Activation Agent | Temperature (K) | CHAdsorption Capacity (mol kg) | CO Adsorption Capacity (mol kg) |
| AC-HPO * | Olive stones | HPO | 303.15 | 6.518 | 10.873 |
| AC-CO * | Olive stones | CO | 303.15 | 3.913 | 5.878 |
| AC-HO * | Olive stones | HO * | 303.15 | 5.417 | 7.968 |
| BC [19] | Babassu coconut | CO | 293 | 5.343 | 10.49 |
| CS [19] | Coconut shell | CO | 293 | 7.259 | 14.67 |
| Pinpel20 [61] | Wood pellets | CO | 303 | 3.36 | 6.66 |
| MSS-AC [62] | Mango Seeds | HPO | 303 | 0.858 | 8.788 |
| CS-HO [39] | Cherry stones | HO | 303 | 8.36 | 14.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peredo-Mancilla, D.; Ghouma, I.; Hort, C.; Ghimbeu, C.M.; Jeguirim, M.; Bessieres, D. CO2 and CH4 Adsorption Behavior of Biomass-Based Activated Carbons. Energies 2018, 11, 3136. https://doi.org/10.3390/en11113136
Peredo-Mancilla D, Ghouma I, Hort C, Ghimbeu CM, Jeguirim M, Bessieres D. CO2 and CH4 Adsorption Behavior of Biomass-Based Activated Carbons. Energies. 2018; 11(11):3136. https://doi.org/10.3390/en11113136
Chicago/Turabian StylePeredo-Mancilla, Deneb, Imen Ghouma, Cecile Hort, Camelia Matei Ghimbeu, Mejdi Jeguirim, and David Bessieres. 2018. "CO2 and CH4 Adsorption Behavior of Biomass-Based Activated Carbons" Energies 11, no. 11: 3136. https://doi.org/10.3390/en11113136
APA StylePeredo-Mancilla, D., Ghouma, I., Hort, C., Ghimbeu, C. M., Jeguirim, M., & Bessieres, D. (2018). CO2 and CH4 Adsorption Behavior of Biomass-Based Activated Carbons. Energies, 11(11), 3136. https://doi.org/10.3390/en11113136