Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability
Abstract
:1. Introduction
- Direct biochar contribution to nutrient availability relies on the macro- and/or micronutrients that biochar contains and their availability [5,9,10]. This is strongly dependent on the composition of the initial feedstock [10] and on the pyrolysis conditions. High retention of alkali species and P is reported for pyrolysis temperatures below 600–700 °C and low heating rates [12,13,14,15], with increase in total, soluble and exchangeable base cations with increasing process severity [13]. However, Uchimiya et al. [16] showed that NaOH-EDTA extractable P increased at 300–350 °C pyrolysis temperature and decreased at higher temperatures for plant and manure biochars. Hossain et al. [17] showed that total N content and N availability decreased with increasing pyrolysis temperature in wastewater sludge biochars.
- Indirect contribution to nutrient availability relies on the capacity of biochars to retain applied nutrients, potentially reducing leaching and increasing fertilizer-use efficiency, on their liming effect or on their impact on other soil properties, such as the potential increase in water-holding capacity, among others, as reviewed by Chan and Xu in [10]. Biochars nutrient retention capacity is associated to their surface charge and surface physical properties as stated by Chan and Xu in [10].
- Biochar surface charge will impact the biochar capacity to adsorb positively or negatively charged nutrients. The cation exchange capacity (CEC) is a measure of the biochar capacity to retain positively charged species, mainly through electrostatic interactions [7,18]. Budai et al. [19] reported that CEC is influenced by different factors, with no clear dominance. These may include: the presence of negatively charged oxygen-containing functional groups on the biochar surface, such as carboxylate (from carboxylic acid) and hydroxyl functional groups [13,18,19,20], which are reported to decrease with increasing conversion temperature [13,18,19,21,22]; the pH of the solution [18]; the content and composition of the inorganic fraction [23,24]. Values for biochars CEC and its dependence on process conditions and feedstock vary significantly in literature [18,19,24]. Biochars anion exchange capacity (AEC) is scarcely reported in literature [20], with studies even showing negligible AEC [18]. Little is known about the origin of AEC on the surface of biochar [20]. However, due to the relevance of this topic, especially towards the reduction in negatively charged nutrients leaching, such as phosphates and nitrates, plenty of recent research activity focuses on the modification of biochar surfaces to enhance their capacity or adsorb such species.
- Biochar specific surface area and pore volume increase with pyrolysis temperature [19,21,25] up to approximately 600–700 °C, when further ordering of the biochar structure may lead to a decrease in surface area [19,25]. Mainly micropores contribute to the specific surface area in biochars [21]. Other process parameters, such as biomass composition, specially inorganic species content, heating rate and pressure can have also a significant impact on porosity development [26,27]. Surface area and pore network may impact biochar adsorption capacity due to the influence on accessibility [18] and availability of adsorption sites.
- With respect to water retention, this depends on both the physical and chemical surface properties of biochar. The nature of surface functional groups impacts the hydrophobic/hydrophilic behavior or biochars [20]. As reported by Schimmelpfenning et al. [21], polar functional groups on the biochar surface may increase the uptake of water due to electrostatic interactions and hydrogen bonds. Their reduction can lead to higher hydrophobic caracter [28]. Hagemann et al. [29] reported that biochar-water interactions enhancement, leading to higher nutrient retention, can be achieved with higher mesoporosity, redox-active sites and hydrophilicity on the surface of biochars.
2. Materials and Methods
Analytical Methods
3. Results and Discussion
3.1. Primary Characterization: Yields, Elemental and Proximate Analysis
3.2. pH and Surface Functional Groups
3.3. Porosity Characterization
3.4. Thermal Stability
3.5. Nutrients
3.6. PAH
3.7. Wettability
4. Conclusions
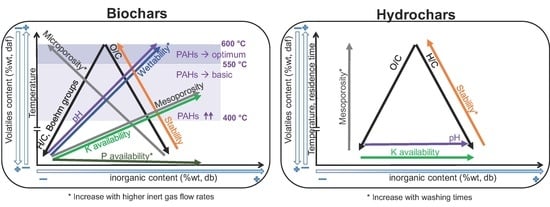
- Regarding primary characterization, biochars O/C molar ratio is linearly correlated to volatiles content, independently of the pyrolysis conditions and feedstock. However, H/C molar ratios of CD biochars are generally lower than those of PW biochars, which may be attributed to the higher inorganic matter content of CD, leading to different pyrolysis reaction pathways and enhancing aromatization. PW hydrochars H/C molar ratio and CD hydrochars O/C molar ratio are also outliers with respect to the linear behavior. This shows that elemental and proximate analysis are not enough to determine the chemical properties of biochars [19], due to the influence of feedstock properties on reaction pathways.
- The pH depends on both conversion process parameters and feedstock, having in general higher pH biochars and hydrochars produced from CD, with much higher inorganic content, including carbonates. In both PW and CD biochars pH correlates strongly with H/C molar ratio. PW biochars and PW hydrochars present also correlation with the O/C ratio. Only CD hydrochars show good correlation (r = 0.88) with the ash content. This shows that the H/C molar ratio may be a better indicator of surface chemistry than O/C ratios or volatiles content, which is also supported by measurements of the total acidic functional groups.
- The porous structure varies greatly among biochars. PW biochars produced at 600 °C present mainly microporosity with N-SSAs around 400 m g. In CD biochars this microporosity is lower (<100 m g). The significantly lower microporosity for all CD biochars is attributed to the high inorganic content, which could potentially block the micropores and enhance mesoporosity. PW and CD biochars produced at 400 °C present also micropores (as shown by CO adsorption), but much less developed than at higher temperatures, i.e., microporosity must be still developed through volatiles release, to open the pores, and further solid aromatization. Furthermore, a higher N sweeping flow during pyrolysis enhances microporosity (more than double for CD biochars at 600 °C). This indicates that micropores blocking is related to volatiles, which may further react to form secondary char or condense in the pores to a greater extent for lower flow rates, when the concentration and retention time of volatiles is higher. Hydrochars, on the contrary, present mainly mesoporosity, independently of the raw material and the process severity, having higher pore volume but generally lower SSA than biochars. Washing of hydrochars further increases the SSA and pore volume due to removal of condensed species.
- Regarding nutrients, NO and NH availability is negligible after pyrolysis, irrespective of feedstock nitrogen content. The absolute availability of PO is reduced compared to the feedstock. For PW biochars the relative availability increases with the pyrolysis conversion. Higher inert gas flow rate leads also to higher PO availability. For hydrochars, HTC can potentially lead to an increase in availability of NO, NH, PO, and K; however, these species are primarily solved in the process water. One exception is PO which appears to form precipitates at higher concentration, i.e., for feedstocks with high direct nutrient potential. Similar to pyrolysis, there is the tendency that the availability of the investigated species is decreased at higher HTC temperatures.
- Biochars have a higher stability to oxidation than hydrochars. Besides the influence of process conditions, PW biochars show higher thermal stability than CD biochars. The same holds true for hydrochars. This may be related to the catalytic effect of AAEM during oxidation reactions.
- PAHs are already produced in biochars at temperatures as low as 340 °C. The maximum quantity is detected for temperatures around 400 °C, exceeding in the case of PW the maximum level for certification. At higher temperatures the PAHs content is reduced again due to vaporization of these species from the solid matrix, achieving a premium quality for temperatures above 550 °C. The composition of these species is highly dependent on the feedstock: naphthalene is the main species in CD biochars, while phenanthrene and fluoranthene are the main species in PW biochars at low temperatures.
- Both PW and CD biochars present mostly a hydrophobic behavior, although it can be reduced with higher conversion temperatures and higher inert gas flow rates during pyrolysis, which reduces the extent of secondary reactions and recondesation of volatiles.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CD | Corn digestate |
| PW | Pine wood |
| HTC | Hydrothermal carbonization |
| PSD | Pore size distribution |
| PAH | Polycyclic aromatic hydrocarbon |
| USEPA | United States Environmental Protection Agency |
References
- European Biochar Certificate (EBC). European Biochar Certificate—Guidelines for Sustainable Production of Biochar; Version 6.2E; European Biochar Foundation (EBC): Arbaz, Switzerland, 2012; Available online: http://www.europeanbiochar.org/en/download (accessed on 4 February 2016).
- Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. International Biochar Intiative Version 2.1. , 2015. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 19 October 2016).
- Abiven, S.; Schmidt, M.; Lehmann, J. Biochar by design. Nat. Geosci. 2014, 7, 326–327. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W. Adsorption of Pb(II) and Cu(II) by Ginkgo-Leaf-Derived Biochar Produced under Various Carbonization Temperatures and Times. Int. J. Environ. Res. Public Health 2017, 14, 1528. [Google Scholar] [CrossRef] [PubMed]
- Frišták, V.; Pipíška, M.; Soja, G. Pyrolysis treatment of sewage sludge: A promising way to produce phosphorus fertilizer. J. Clean. Prod. 2018, 172, 1772–1778. [Google Scholar] [CrossRef]
- Frišták, V.; Pipíška, M.; Lesný, J.; Soja, G.; Friesl-Hanl, W.; Packová, A. Utilization of biochar sorbents for Cd2+, Zn2+, and Cu2+ ions separation from aqueous solutions: Comparative study. Environ. Monit. Assess. 2014, 187, 4093. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Charles, U.P., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Chapter 2—A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 105, pp. 47–82. [Google Scholar]
- Chan, K.Y.; Xu, Z. Nutrient Properties and Their Enhancement. In Biochar for Environmental Management. Science and Technology; Earthscan: Abingdon, UK, 2009; Chapter 5; pp. 67–84. ISBN 978-1-84407-658-1. [Google Scholar]
- Thies, J.E.; Rillig, M.C. Characteristics of Biochar: Biological Properties. In Biochar for Environmental Management. Science and Technolgy; Earthscan: Abingdon, UK, 2009; Chapter 6; pp. 85–105. [Google Scholar]
- Anca-Couce, A.; Dieguez-Alonso, A.; Zobel, N.; Berger, A.; Kienzl, N.; Behrendt, F. Influence of Heterogeneous Secondary Reactions during Slow Pyrolysis on Char Oxidation Reactivity of Woody Biomass. Energy Fuels 2017, 31, 2335–2344. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Angst, T.E.; Sohi, S.P. Establishing release dynamics for plant nutrients from biochar. GCB Bioenergy 2013, 5, 221–226. [Google Scholar] [CrossRef]
- Zambon, I.; Colosimo, F.; Monarca, D.; Cecchini, M.; Gallucci, F.; Proto, R.A.; Lord, R.; Colantoni, A. An Innovative Agro-Forestry Supply Chain for Residual Biomass: Physicochemical Characterisation of Biochar from Olive and Hazelnut Pellets. Energies 2016, 9, 526. [Google Scholar] [CrossRef] [Green Version]
- Uchimiya, M.; Hiradate, S.; Antal, M.J. Dissolved Phosphorus Speciation of Flash Carbonization, Slow Pyrolysis, and Fast Pyrolysis Biochars. ACS Sustain. Chem. Eng. 2015, 3, 1642–1649. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Zimmerman, A.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Budai, A.; Wang, L.; Gronli, M.; Strand, L.T.; Antal, M.J.; Abiven, S.; Dieguez-Alonso, A.; Anca-Couce, A.; Rasse, D.P. Surface Properties and Chemical Composition of Corncob and Miscanthus Biochars: Effects of Production Temperature and Method. J. Agric. Food Chem. 2014, 62, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Lawrinenko, M.; Laird, D.A. Anion exchange capacity of biochar. Green Chem. 2015, 17, 4628–4636. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One step forward toward characterization: Some important material properties to distinguish biochars. J. Environ. Qual. 2011, 42, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Carrier, M.; Hardie, A.G.; Uras, A.; Görgens, J.; Knoetze, J.H. Production of char from vacuum pyrolysis of South-African sugar cane bagasse and its characterization as activated carbon and biochar. J. Anal. Appl. Pyrolysis 2012, 96, 24–32. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.; Moghtaderi, B.; Gupta, R.; Wall, T. Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 2004, 83, 2139–2150. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical Properties of Biochar. In Biochar for Environmental Management. Science and Technology; Earthscan: Abingdon, UK, 2009; Chapter 2; pp. 13–32. ISBN 978-1-84407-658-1. [Google Scholar]
- Liu, Z.; Zhang, F.S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- McCormack, S.A.; Ostle, N.; Bardgett, R.D.; Hopkins, D.W.; Vanbergen, A.J. Biochar in bioenergy cropping systems: Impacts on soil faunal communities and linked ecosystem processes. GCB Bioenergy 2013, 5, 81–95. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, H.J.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Huff, M.D.; Kumar, S.; Lee, J.W. Comparative analysis of pinewood, peanut shell, and bamboo biomass derived biochars produced via hydrothermal conversion and pyrolysis. J. Environ. Manag. 2014, 146, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ro, K.S.; Chappell, M.; Li, Y.; Mao, J. Chemical Structures of Swine-Manure Chars Produced under Different Carbonization Conditions Investigated by Advanced Solid-State 13C Nuclear Magnetic Resonance (NMR) Spectroscopy. Energy Fuels 2011, 25, 388–397. [Google Scholar] [CrossRef]
- Wiedner, K.; Naisse, C.; Rumpel, C.; Pozzi, A.; Wieczorek, P.; Glaser, B. Chemical modification of biomass residues during hydrothermal carbonization—What makes the difference, temperature or feedstock? Org. Geochem. 2013, 54, 91–100. [Google Scholar] [CrossRef]
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Busch, D.; Glaser, B. Stability of co-composted hydrochar and biochar under field conditions in a temperate soil. Soil Use Manag. 2015, 31, 251–258. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Anca-Couce, A.; Zobel, N. On-line tar characterization from pyrolysis of wood particles in a technical-scale fixed-bed reactor by applying Laser-Induced Fluorescence (LIF). J. Anal. Appl. Pyrolysis 2013, 102, 33–46. [Google Scholar] [CrossRef]
- Naumann, C.; Bassler, R.; Seibold, R.; Barth, C. Methodenbuch. Band III, Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 1997. [Google Scholar]
- Boehm, H.P.; Diehl, E.; Heck, W.; Sappok, R. Surface Oxides of Carbon. Angew. Chem. Int. Ed. 1964, 3, 669–677. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Theriault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Tsechansky, L.; Graber, E.R. Methodological limitations to determining acidic groups at biochar surfaces via the Boehm titration. Carbon 2014, 66, 730–733. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Seaton, N.; Walton, J.; Quirke, N. A new analysis method for the determination of the pore-size distribution of porous carbons from nitrogen adsorption measurements. Carbon 1989, 27, 853–861. [Google Scholar] [CrossRef]
- Hoffmann, G. Methodenbuch. Band I, Die Untersuchung von Böden; VDLUFA-Verlag: Darmstadt, Germany, 1991. [Google Scholar]
- Fabbri, D.; Rombola, A.G.; Torri, C.; Spokas, K.A. Determination of polycyclic aromatic hydrocarbons in biochar and biochar amended soil. J. Anal. Appl. Pyrolysis 2013, 103, 60–67. [Google Scholar] [CrossRef]
- Ojeda, G.; Mattana, S.; Avila, A.; Alcaniz, J.M.; Volkmann, M.; Bachmann, J. Are soil-water functions affected by biochar application? Geoderma 2015, 249–250, 1–11. [Google Scholar] [CrossRef]
- Krull, E.S.; Baldock, J.A.; Skjemstad, J.O.; Smernik, R.J. Characteristics of Biochar: Organo-Chemical Properties. In Biochar for Environmental Management. Science and Technology; Earthscan: Abingdon, UK, 2009; Chapter 4; pp. 53–65. ISBN 978-1-84407-658-1. [Google Scholar]
- Rombolà, A.G.; Fabbri, D.; Meredith, W.; Snape, C.E.; Dieguez-Alonso, A. Molecular characterization of the thermally labile fraction of biochar by hydropyrolysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2016, 121, 230–239. [Google Scholar] [CrossRef]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cao, X.; Zhao, L.; Wang, J.; Ding, Z. Effects of Mineral Additives on Biochar Formation: Carbon Retention, Stability, and Properties. Environ. Sci. Technol. 2014, 48, 11211–11217. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Mehrotra, A.K.; Tan, Z. Alkaline hydrothermal conversion of cellulose to bio-oil: Influence of alkalinity on reaction pathway change. Bioresour. Technol. 2011, 102, 6605–6610. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Novicio, L.; Hata, T.; Kurimoto, Y.; Doi, S.; Ishihara, S.; Imamura, Y. Adsorption capacities and related characteristics of wood charcoals carbonized using a one-step or two-step process. J. Wood Sci. 2001, 47, 48–57. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J. Black carbon decomposition under varying water regimes. Org. Geochem. 2009, 40, 846–853. [Google Scholar] [CrossRef]
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Lee, J.W.; Kidder, M.; Evans, B.R.; Paik, S.; Buchanan, A.C., III; Garten, C.T.; Brown, R.C. Characterization of Biochars Produced from Cornstovers for Soil Amendment. Environ. Sci. Technol. 2010, 44, 7970–7974. [Google Scholar] [CrossRef] [PubMed]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The Interfacial Behavior between Biochar and Soil Minerals and Its Effect on Biochar Stability. Entviron. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Harvey, O.R.; Kuo, L.J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars). Environ. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.J.; Herbert, B.E.; Louchouarn, P. Can levoglucosan be used to characterize and quantify char/charcoal black carbon in environmental media? Org. Geochem. 2008, 39, 1466–1478. [Google Scholar] [CrossRef]
- Plante, A.F.; Fernández, J.M.; Haddix, M.L.; Steinweg, J.M.; Conant, R.T. Biological, chemical and thermal indices of soil organic matter stability in four grassland soils. Soil Biol. Biochem. 2011, 43, 1051–1058. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Funke, A. Fate of Plant Available Nutrients during Hydrothermal Carbonization of Digestate. Chem. Ing. Tech. 2015, 87, 1713–1719. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Funke, A.; Mumme, J.; Koon, M.; Diakité, M. Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy 2013, 58, 229–237. [Google Scholar] [CrossRef]
- Kruse, A.; Koch, F.; Stelzl, K.; Wüst, D.; Zeller, M. Fate of Nitrogen during Hydrothermal Carbonization. Energy Fuels 2016, 30, 8037–8042. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Anca-Couce, A.; Zobel, N.; Behrendt, F. Understanding the primary and secondary slow pyrolysis mechanisms of holocellulose, lignin and wood with laser-induced fluorescence. Fuel 2015, 153, 102–109. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; MacKinnon, G.; Masek, O. Strategies for producing biochars with minimum {PAH} contamination. J. Anal. Appl. Pyrolysis 2016, 119, 24–30. [Google Scholar] [CrossRef]









| Authors | Process Conditions | Feedstock | Methods |
|---|---|---|---|
| Budai et al. [19] | Biochars from slow pyrolysis (235–800 °C), flash carbonization char (600 °C) and hydrochars (230 °C for 6 h) | Corncob and miscanthus | Proximate and elemental (CHNO) analyses, pH, CEC, SSA with N adsorption |
| Schimmelpfennig and Glaser [21] | Biochars from slow pyrolysis (Pyreg unit) (550 °C), hydrochars (200 °C), rotary kiln biochars (350–550, 750 °C), gasification biochars (800 °C), traditional kiln biochars (350 °C), other biochars (350, 500, 650, 800 °C) | Wood, animal meal, sugar cane/beet, wheat, bamboo, maize, rice hulls, peanut shells, sewage sludge, walnut shells, girasol, coconut shells, lop, bark/needles | Elemental (CHNO) analysis, black carbon content, SSA with N adsorption, PAHs content |
| Liu et al. [28] | Biochars from slow pyrolysis (700 °C), hydrochars (300 °C for 20 min) | Pine wood | Proximate and elemental (CHNO) analyses, Boehm titration, pH, FTIR spectroscopy, surface morphology with SEM, SSA and micro-, meso- and macropores volume with N adsorption, copper removal capacity (adsorption) from wastewater |
| Huff et al. [35] | Biochars from slow pyrolysis (300, 400 and 500 °C) and hydrochars (300 °C for 30 min) | Pine wood, peanut shell and bamboo | Proximate and elemental (CHN) analyses, methylene blue adsorption, CEC, FTIR spectroscopy |
| Cao et al. [36] | Biochars from slow pyrolysis (620 °C) and hydrochars (with acid prewashing and water and acetone washing) (250 °C for 20 h) | Swine-manure | Major chemical structural components with Quantitative C Direct Polarization/Magic-Angle Spinning (DP/MAS) NMR, C Cross-Polarization/Total Suppression of Sidebands (CP/TOSS) and C CP/TOSS Plus Dipolar Dephasing, C Chemical-Shift-Anisotropy (CSA) Filter. Connectivities of different functional groups in hydrochars with H–C Two-Dimensional Heteronuclear Correlation (2D HETCOR) NMR. Aromatic cluster sizes with H–C Long-Range Recoupled H–C Dipolar Dephasing Experiments |
| Wiedner et al. [37] | Biochars (pyrolysis-Pyreg up to 850 °C, pyrolysis/ gasification-BC up to 760 °C, gasification-AGT up to 1200 °C, gasification-CT up to 550 °C) and hydrochars (1st stage at 230 °C for at least 15 min and 2nd stage at 180 °C for at least 75 min; 170 °C for 90 min) | Wheat straw-AGT, wood chips-AGT, poplar-AGT, sorghum-AGT, olive residues-AGT, wood chips-CT, wood chips-BC, draff-Pyreg, miscanthus-Pyreg. Maize silage, leftover food, biogas digestate, grass greenery and sewage sludge for hydorchars | pH, EC, ash content, elemental composition (CHNO), C NMR, SEM-EDS, black carbon content, PAHs content, polychlorinated dibenzodioxines (PCDDs) and polychlorinated dibenzofurans (PCDFs) content |
| Busch et al. [38] | Biochars (gasification-AGT up to 1200 °C and pyrolysis at 400–600 °C) and hydrochars (230 °C for 1 h and 180 °C for at least 4 h; 170 °C) | Olive residues-AGT, poplar wood chips-AGT, wheat straw-AGT, miscanthus-pyrolysis. Maize silage, food leftovers, biogas digestate grass and sewage eludge for hydrochars | Tradescantia genotoxicity assay, plant germination and growth tests and impact on soil pH |
| Busch and Glasser [39] | Biochar (pyrolysis-Pyreg), hydrochar (230 °C for 1 h and 180 °C for at least 4 h), co-composted biochar (1:1 w/w) and hydrochar (1:3 w/w) with raw biomass residues from vegetable waste from horticulture and landscaping, kept for 4 weeks in temperature range 55–75 °C) | Conifer wood bark residues for biochar and maize silage for hydrochar | Stability of co-composted hydrochar and biochar under field conditions in a temperate soil. TOC, total N content, pH, EC and black carbon analysis of biochars, hydrochars, compost and co-composted biochars and hydrochars before and after application in soil |
| Material | Ash (%, db) | C (%, db) | H (%, db) | O (%, db) | N (%, db) | |
|---|---|---|---|---|---|---|
| CD | 19.6 ± 0.1 | 40.7 ± 0.7 | 5.5 ± 0.1 | 31.7 ± 0.8 | 2.4 ± 0.5 | |
| PW | 0.2 ± 0.0 | 49.4 ± 0.4 | 6.7 ± 0.1 | 43.7 ± 0.4 | 0.1 ± 0.0 | |
| Sample | Raw | Process | HTT * | Flow | Char yield | |
| material | (°C) | (NL min) | (%, db) | |||
| PW400-20 | Pine wood | Pyrolysis | 400 | 20 | 30.01 ± 0.18 | |
| PW400-40 | Pine wood | Pyrolysis | 400 | 40 | 25.83 ± 2.12 | |
| PW600-20 | Pine wood | Pyrolysis | 600 | 20 | 24.94 | |
| PW600-40 | Pine wood | Pyrolysis | 600 | 40 | 22.82 ± 0.49 | |
| CD400-20 | Corn digest. | Pyrolysis | 400 | 20 | 50.89 ± 0.36 | |
| CD400-40 | Corn digest. | Pyrolysis | 400 | 40 | 52.00 ± 0.21 | |
| CD600-20 | Corn digest. | Pyrolysis | 600 | 20 | 49.76 ± 0.40 | |
| CD600-40 | Corn digest. | Pyrolysis | 600 | 40 | 48.54 ± 1.08 | |
| Sample | Raw | Process | HTT | Time | Wash. | Char yield |
| material | (°C) | (min) | times | (%, db) | ||
| PW200-10 | Pine wood | HTC | 200 | 10 | - | 82.30 ± 0.35 |
| PW200-360 | Pine wood | HTC | 200 | 360 | - | 75.68 ± 0.01 |
| PW240-10 | Pine wood | HTC | 240 | 10 | - | 76.26 ± 0.07 |
| PW240-360 | Pine wood | HTC | 240 | 360 | - | 59.09 ± 0.19 |
| CD200-10-0 | Corn digest. | HTC | 200 | 10 | 0 | 83.49 |
| CD200-10-6 | Corn digest. | HTC | 200 | 10 | 6 | 73.49 |
| CD200-360-0 | Corn digest. | HTC | 200 | 360 | 0 | 74.38 |
| CD200-360-6 | Corn digest. | HTC | 200 | 360 | 6 | 70.29 |
| CD220-185-3 | Corn digest. | HTC | 220 | 185 | 3 | 64.57 ± 2.02 |
| CD240-10-0 | Corn digest. | HTC | 240 | 10 | 0 | 70.82 |
| CD240-10-6 | Corn digest. | HTC | 240 | 10 | 6 | 66.85 |
| CD240-360-0 | Corn digest. | HTC | 240 | 360 | 0 | 62.06 |
| R | C (% mass, daf) | H/C (molar) | O/C (molar) | |
|---|---|---|---|---|
| PW200-360 | 0.43 (0.41) | 56.94 | 0.96 | 0.50 |
| PW240-10 | 0.41(0.39) | 55.77 | 1.00 | 0.53 |
| PW240-360 | 0.51 (0.48) | 70.33 | 0.67 | 0.27 |
| CD200-360-0 | 0.41 (0.39) | 62.93 | 1.19 | 0.33 |
| CD200-360-6 | 0.41 (0.39) | 62.86 | 1.22 | 0.33 |
| CD240-10-0 | 0.43 (0.41) | 61.69 | 1.29 | 0.35 |
| CD240-10-6 | 0.42 (0.40) | 61.77 | 1.32 | 0.35 |
| CD240-360-0 | 0.45 (0.43) | 76.35 | 1.11 | 0.12 |
| PW400-20 | 0.53 (0.50) | 82.10 | 0.54 | 0.13 |
| PW600-20 | 0.57 (0.54) | 93.49 | 0.22 | 0.04 |
| CD600-20 | 0.52 (0.50) | 84.62 | 0.18 | 0.09 |
| CD600-40 | 0.52 (0.50) | 81.99 | 0.19 | 0.13 |
| C/N * | N (db) | Mg (db) | Ca (db) | P (db) | K (db) | Mg | Ca | P | NH | NO | PO | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | % | mg/kg | mg/kg | mg/kg | mg/kg | - | - | - | mg/kg | mg/kg | mg/kg | mg/kg | |
| PW | 912.7 | 0.1 | 178.1 ± 38.7 | 894.5 ± 305.7 | - | 322.1 ± 81.6 | - | - | - | 2.3 | 0.9 | 13 | 230 |
| CD | 20.1 | 2.4 | - | - | 13,900 ± 440 | 9600 ± 360 | - | - | - | 1200 ± 110 | 1800 ± 130 | 1800 ± 160 | 6100 ± 350 |
| PW400-20 | 550.9 ± 57.9 | 0.2 ± 0.0 | - | - | - | - | - | - | - | n.d. | 0.9 | 27 ± 9 | 300 ± 30 |
| PW400-40 | 729.2 ± 54.2 | 0.1 ± 0.0 | - | - | - | - | - | - | - | n.d. | n.d. | 37 ± 2 | 370 ± 60 |
| PW600-20 | 634.1 ± 106.8 | 0.2 ± 0.0 | - | - | - | - | - | - | - | 0.9 ± 0.2 | n.d. | 16 ± 2 | 900 ± 40 |
| PW600-40 | 694.3 ± 91.3 | 0.2 ± 0.0 | - | - | - | - | - | - | - | 0.4 ± 0.1 | 0.6 | 43 ± 6 | 750 ± 40 |
| CD400-20 | 22.6 ± 3.5 | 2.4 ± 0.4 | 20,568.0 ± 2233.3 | 139,051.2 ± 23,155.2 | 26,458.0 ± 2163.8 | 11,882.6 ± 1303.1 | - | - | - | 1.2 ± 0.5 | n.d. | 1360 ± 110 | 4610 ± 1680 |
| CD400-40 | 22.4 ± 0.2 | 2.3 ± 0.1 | 22,608.3 ± 3140.2 | 154,160.6 ± 25,380.1 | 30,319.7 ± 3287.0 | 13,184.2 ± 1594.7 | - | - | - | 2.6 | n.d. | 1650 | 7460 |
| CD600-20 | 29.4 ± 1.5 | 1.8 ± 0.1 | 21,796.2 ± 1781.4 | 135,010.5 ± 15,103.8 | 27,975.3 ± 2270.6 | 12,410.9 ± 1441.5 | - | - | - | 1.4 ± 0.4 | 11 | 960 ± 130 | 7890 ± 1580 |
| CD600-40 | 29.6 ± 1.4 | 1.7 ± 0.1 | 22,150.4 ± 2930.0 | 156,042.0 ± 13,715.9 | 29,015.1 ± 3091.5 | 12,601.1 ± 1442.5 | - | - | - | 1.3 ± 0.1 | n.d. | 1510 ± 50 | 8210 ± 1410 |
| [-] | % | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| PW200-10 | 522.8 ± 80.7 | 0.1 ± 0.0 | 95.8 ± 9.8 | 733.1 ± 54.3 | 61.5 ± 4.2 | 213.1 ± 19.5 | 60.0 ± 8.8 | 126.7 ± 38.3 | 31.7 ± 9.4 | 5.1 ± 0.2 | 5.3 ± 0.5 | 24.2 ± 7.5 | 124 ± 20 |
| PW200-360 | 841.7 ± 68.6 | 0.1 ± 0.0 | 99.3 ± 14.8 | 446.6 ± 14.1 | 78.7 ± 31.3 | 255.4 ± 9.6 | 48.2 ± 8.7 | 145.1 ± 10.7 | 16.0 ± 1.1 | 1.1 ± 0.1 | 5.5 ± 1.9 | 11.4 ± 1.1 | 112 ± 2 |
| PW240-10 | 616.1 ± 248.0 | 0.1 ± 0.1 | 72.0 ± 0.1 | 500.1 ± 4.0 | 48.9 ± 3.0 | 183.4 ± 9.3 | 42.8 ± 0.4 | 135.6 ± 1.2 | 22.3 ± 0.1 | 2.1 ± 0.2 | 5.9 ± 0.0 | 17.4 ± 0.6 | 115 ± 3 |
| PW240-360 | 1802.3 ± 1351.3 | 0.1 ± 0.1 | 118.4 ± 4.6 | 624.8 ± 16.7 | 71.2 ± 1.3 | 336.0 ± 31.1 | 40.9 ± 1.0 | 143.3±1.2 | 10.4 ± 1.1 | 1.2 ± 0.3 | 5.0 ± 3.3 | 3.5 ± 0.6 | 121 ± 6 |
| CD200-10-0/6 | 28.8 ± 2.1 | 1.9 ± 0.1 | 9565.4 | 44,518.8 ± 5535.0 | 15,735.0 ± 1087.1 | 5359.6 ± 2479.4 | 3.5 | 1027.5 ± 298.6 | 341.2 ± 131.4 | 230 ± 30 | 19.2 ± 4.2 | 190 ± 48 | 1490 ± 20 |
| CD200-360-0/6 | 27.3 ± 1.8 | 2.0 ± 0.1 | 11,292.0 | 54,222.0 ± 9737.7 | 18,429.3 ± 602.5 | 4226.9 ± 2928.9 | 189.0 ± 263.3 | 2356.9 ± 439.9 | 63.9 ± 19.1 | 260 ± 65 | 16.0 ± 4.1 | 60 ± 31 | 2180 ± 160 |
| CD220-185-3 | 25.6 ± 0.5 | 2.2 ± 0.0 | 12,970.2 | 53,535.0± 1943.2 | 19,555.3 ± 671.9 | 2141.8 ± 492.8 | 3.1 | 1985.0 ± 99.0 | 60.8 ± 6.0 | 180 ± 62 | 8.3 ± 2.3 | 51 ± 8 | 1890 ± 360 |
| CD240-10-0/6 | 27.3 ± 1.9 | 1.8 ± 0.1 | 10,753.2 ± 1330.7 | 101,258.3 ± 50,630.1 | 15,962.0 ± 435.4 | 3158.0 ± 1476.1 | 473.3 ± 618.1 | 2301.6 ± 530.4 | 112.9 ± 84.6 | 360 ± 81 | 8.1 ± 1.9 | 100 ± 80 | 1870 ± 240 |
| CD240-360-0 | 22.5 | 2.5 | - | 110,882.0 | 20,862.4 | 4656.2 | - | 2172 .0 | 50.5 | 140 | 1.6 | 32 | 1470 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies 2018, 11, 496. https://doi.org/10.3390/en11030496
Dieguez-Alonso A, Funke A, Anca-Couce A, Rombolà AG, Ojeda G, Bachmann J, Behrendt F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies. 2018; 11(3):496. https://doi.org/10.3390/en11030496
Chicago/Turabian StyleDieguez-Alonso, Alba, Axel Funke, Andrés Anca-Couce, Alessandro Girolamo Rombolà, Gerardo Ojeda, Jörg Bachmann, and Frank Behrendt. 2018. "Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability" Energies 11, no. 3: 496. https://doi.org/10.3390/en11030496
APA StyleDieguez-Alonso, A., Funke, A., Anca-Couce, A., Rombolà, A. G., Ojeda, G., Bachmann, J., & Behrendt, F. (2018). Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies, 11(3), 496. https://doi.org/10.3390/en11030496