Integrated Leaching and Thermochemical Technologies for Producing High-Value Products from Rice Husk: Leaching of Rice Husk with the Aqueous Phases of Bioliquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
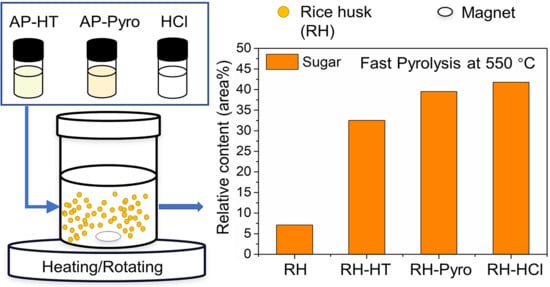
2.2. Preparation of Leaching Reagents and the Leaching Process
2.3. Analytical Methods
3. Results
3.1. Acidity of AP-HT Benchmarking against that of AP-Pyro and Their Compositions
3.2. Leaching Effect of AP-HT and AP-Pyro Benchmarking against that of HCl on AAEMs Removal from RH
3.3. Reaction Pathway of the AAEMs Removal from RH by the Leaching Agents
3.4. Effects of AP Leaching on the Pyrolysis Characteristics and the Liquid Products
3.5. Further Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Panwar, N.; Kaushik, S.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Song, B.; Lin, R.; Lam, C.H.; Wu, H.; Tsui, T.-H.; Yu, Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew. Sustain. Energy Rev. 2021, 135, 110370. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Yang, L.; Ding, K.; Zhang, Y.; Gao, W.; Huang, Y.; Sun, H.; Hu, X.; Syed-Hassan, S.S.A.; Zhang, S.; et al. Mini-Review on Char Catalysts for Tar Reforming during Biomass Gasification: The Importance of Char Structure. Energy Fuels 2019, 34, 1219–1229. [Google Scholar] [CrossRef]
- Beims, R.F.; Hu, Y.; Shui, H.; Xu, C.C. Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Products applications and challenges to develop large-scale operations. Biomass-Bioenergy 2020, 135, 105510. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Y.; Zhou, H.; Sun, H.; Zhou, J.; Zhang, S. Pyrolysis of palm kernel shell with internal recycling of heavy oil. Bioresour. Technol. 2019, 272, 77–82. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Seljak, T.; Buffi, M.; Valera-Medina, A.; Chong, C.; Chiaramonti, D.; Katrašnik, T. Bioliquids and their use in power generation—A technology review. Renew. Sustain. Energy Rev. 2020, 129, 20. [Google Scholar] [CrossRef]
- Nava, R.; Pawelec, B.; Castaño, P.; Álvarez-Galván, M.; Loricera, C.; Fierro, J.L.G. Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl. Catal. B Environ. 2009, 92, 154–167. [Google Scholar] [CrossRef]
- Jia, C.; Chen, J.; Liang, J.; Song, S.; Liu, K.; Jiang, A.; Wang, Q. Pyrolysis characteristics and kinetic analysis of rice husk. J. Therm. Anal. Calorim. 2020, 139, 577–587. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Li, J.; Wang, Q.; He, Z.; Feng, Y.; Abomohra, A.E.-F.; Afonaa-Mensah, S.; Hui, C. Co-pyrolysis and co-hydrothermal liquefaction of seaweeds and rice husk: Comparative study towards enhanced biofuel production. J. Anal. Appl. Pyrolysis 2018, 129, 162–170. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Ahmad, A.; Giri, B.S.; Chaturvedi, P. Hydrothermal liquefaction of rice husk and cow dung in Mixed-Bed-Rotating Pyrolyzer and application of biochar for dye removal. Bioresour. Technol. 2020, 309, 123294. [Google Scholar] [CrossRef] [PubMed]
- Weldekidan, H.; Strezov, V.; Town, G.E.; Kan, T. Production and analysis of fuels and chemicals obtained from rice husk pyrolysis with concentrated solar radiation. Fuel 2018, 233, 396–403. [Google Scholar] [CrossRef]
- Deng, W.; Xu, K.; Xiong, Z.; Chaiwat, W.; Wang, X.; Su, S.; Hu, S.; Qiu, J.; Wangab, Y.; Xiang, J. Evolution of Aromatic Structures during the Low-Temperature Electrochemical Upgrading of Bio-oil. Energy Fuels 2019, 33, 11292–11301. [Google Scholar] [CrossRef]
- The Interagency Commodity Estimates Committees. World Agricultural Supply and Demand Estimates; United States Department of Agriculture: Washington, DC, USA, 2019.
- Lim, J.S.; Manan, Z.A.; Alwi, S.R.W.; Hashim, H. A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Nik-Azar, M.; Hajaligol, M.; Sohrabi, M.; Dabir, B. Mineral matter effects in rapid pyrolysis of beech wood. Fuel Process. Technol. 1997, 51, 7–17. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Qiao, Y.; Lin, J.-Y.; Wang, M.; Chang, L. Effect of atmosphere on the release behavior of alkali and alkaline earth metals during coal oxy-fuel combustion. Fuel 2015, 139, 164–170. [Google Scholar] [CrossRef]
- Lam, C.H.; Deng, W.; Lang, L.; Jin, X.; Hu, X.; Wang, Y. Minireview on Bio-Oil Upgrading via Electrocatalytic Hydrogenation: Connecting Biofuel Production with Renewable Power. Energy Fuels 2020, 34, 7915–7928. [Google Scholar] [CrossRef]
- Han, Y.; Gholizadeh, M.; Tran, C.-C.; Kaliaguine, S.; Li, C.-Z.; Olarte, M.; Garcia-Perez, M. Hydrotreatment of pyrolysis bio-oil: A review. Fuel Process. Technol. 2019, 195, 106140. [Google Scholar] [CrossRef]
- Liaw, S.B.; Wu, H. Leaching Characteristics of Organic and Inorganic Matter from Biomass by Water: Differences between Batch and Semi-continuous Operations. Ind. Eng. Chem. Res. 2013, 52, 4280–4289. [Google Scholar] [CrossRef]
- Asadieraghi, M.; Daud, W.M.A.W. Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: Effects of demineralization by diverse acid solutions. Energy Convers. Manag. 2014, 82, 71–82. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Sun, L.-S.; Su, S.; Xu, K.; He, L.-M.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, Y.; Cen, K.; Cao, X.; Ma, Z.; Li, Y. Comparative study on the pyrolysis behaviors of rice straw under different washing pretreatments of water, acid solution, and aqueous phase bio-oil by using TG-FTIR and Py-GC/MS. Fuel 2019, 252, 1–9. [Google Scholar] [CrossRef]
- De Caprariis, B.; De Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Özdenkçi, K.; Muddassar, H.R.; Melin, K.; Golam, S.; Oinas, P. Hydrothermal Liquefaction of Softwood: Selective Chemical Production Under Oxidative Conditions. ACS Sustain. Chem. Eng. 2016, 4, 3978–3984. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Hantoko, D.; Susanto, H.; Ardy, A.; Waluyo, J.; Weng, Z.; Lin, J. Hydrothermal treatment of empty fruit bunch and its pyrolysis characteristics. Biomass-Convers. Biorefinery 2019, 9, 709–717. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Chen, F.; Ma, Z.; Zhou, J.; Li, M. Are the typical organic components in biomass pyrolyzed bio-oil available for leaching of alkali and alkaline earth metallic species (AAEMs) from biomass? Fuel 2020, 260, 116347. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Templeton, D. Determination of Ash in Biomass, Laboratory Analytical Procedure.; Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Karnowo; Zahara, Z.F.; Kudo, S.; Norinaga, K.; Hayashi, J.-I. Leaching of Alkali and Alkaline Earth Metallic Species from Rice Husk with Bio-oil from Its Pyrolysis. Energy Fuels 2014, 28, 6459–6466. [Google Scholar] [CrossRef]
- Salo, K.; Mojtahedi, W. Fate of alkali and trace metals in biomass gasification. Biomass Bioenergy 1998, 15, 263–267. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.; Faucher, M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wu, Y.; Han, Y.; Qin, C.; Nie, S.; Liu, S.; Wang, S.; Yao, S. Effect of hydrothermal pretreatment on the demineralization and thermal degradation behavior of eucalyptus. Bioresour. Technol. 2020, 307, 123246. [Google Scholar] [CrossRef] [PubMed]
- Taboada, E.; Fisher, P.; Jara, R.; Zuniga, E.; Gidekel, M.; Cabrera, J.C.; Pereira, E.; Gutierrez-Moraga, A.; Villalonga, R.; Cabrera, G. Isolation and characterisation of pectic substances from murta (Ugni molinae Turcz) fruits. Food Chem. 2010, 123, 669–678. [Google Scholar] [CrossRef]
- Oh, B.-C.; Kim, M.H.; Yun, B.-S.; Choi, W.-C.; Park, S.-C.; Bae, S.-C.; Oh, T.-K. Ca2+-inositol phosphate chelation mediates the substrate specificity of beta-propeller phytase. Biochemistry 2006, 45, 9531–9539. [Google Scholar] [CrossRef] [PubMed]
- Connatser, R.M.; Frith, M.G.; Jun, J.; Lewis, S.A.; Brady, M.P.; Keiser, J.R. Approaches to investigate the role of chelation in the corrosivity of biomass-derived oils. Biomass Bioenergy 2020, 133, 105446. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Liang, D.T.; Zheng, C. Mechanism of palm oil waste pyrolysis in a packed bed. Energy Fuels 2006, 20, 1321–1328. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Quantitative Structures and Thermal Properties of Birch Lignins after Ionic Liquid Pretreatment. J. Agric. Food Chem. 2013, 61, 635–645. [Google Scholar] [CrossRef]
- Park, B.-D.; Wi, S.G.; Lee, K.H.; Singh, A.P.; Yoon, T.-H.; Kim, Y.S. Characterization of anatomical features and silica distribution in rice husk using microscopic and micro-analytical techniques. Biomass Bioenergy 2003, 25, 319–327. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Effects of various solvents on the liquefaction of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2008, 49, 3498–3504. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Zhou, H.; Meng, Y.; Wang, S. Characterization of adhesive penetration in wood bond by means of scanning thermal microscopy (SThM). Holzforschung 2016, 70, 323–330. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Wang, S.; Dong, L.; Li, C.-Z. Effects of Alkali and Alkaline Earth Metallic Species and Chemical Structure on Nascent Char-O-2 Reactivity. Energy Fuels 2017, 31, 13578–13584. [Google Scholar] [CrossRef]
- Mahadevan, R.; Adhikari, S.; Shakya, R.; Wang, K.; Dayton, D.; Lehrich, M.; Taylor, S.E. Effect of Alkali and Alkaline Earth Metals on in-Situ Catalytic Fast Pyrolysis of Lignocellulosic Biomass: A Microreactor Study. Energy Fuels 2016, 30, 3045–3056. [Google Scholar] [CrossRef]
- Guo, F.H.; Zhong, Z.P. Optimization of the co-combustion of coal and composite biomass pellets. J. Clean. Prod. 2018, 185, 399–407. [Google Scholar] [CrossRef]
- Mourant, D.; Wang, Z.; He, M.; Wang, X.S.; Garcia-Perez, M.; Ling, K.; Li, C.-Z. Mallee wood fast pyrolysis: Effects of alkali and alkaline earth metallic species on the yield and composition of bio-oil. Fuel 2011, 90, 2915–2922. [Google Scholar] [CrossRef]
- Eom, I.-Y.; Kim, J.-Y.; Kim, T.-S.; Lee, S.-M.; Choi, D.; Choi, I.-G.; Choi, J.-W. Effect of essential inorganic metals on primary thermal degradation of lignocellulosic biomass. Bioresour. Technol. 2012, 104, 687–694. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, Y. Washing pretreatment with light bio-oil and its effect on pyrolysis products of bio-oil and biochar. RSC Adv. 2016, 6, 5270–5277. [Google Scholar] [CrossRef]
- Stankovikj, F.; McDonald, A.G.; Helms, G.L.; Olarte, M.V.; Garcia-Perez, M. Characterization of the Water-Soluble Fraction of Woody Biomass Pyrolysis Oils. Energy Fuels 2017, 31, 1650–1664. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Chen, Z.; Yoshikawa, K. Clean solid biofuel production from high moisture content waste biomass employing hydrothermal treatment. Appl. Energy 2014, 131, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Medrano, J.; Oliva, M.; Ruiz, J.; García, L.; Arauzo, J. Hydrogen from aqueous fraction of biomass pyrolysis liquids by catalytic steam reforming in fluidized bed. Energy 2011, 36, 2215–2224. [Google Scholar] [CrossRef]
- Li, H.; Xu, Q.; Xue, H.; Yan, Y. Catalytic reforming of the aqueous phase derived from fast-pyrolysis of biomass. Renew. Energy 2009, 34, 2872–2877. [Google Scholar] [CrossRef]







| Sample | Proximate Analysis (wt%, ar) | Ultimate Analysis (wt%, daf) | |||||||
| Water Content | Volatile matter | Fixed Carbon a | Ash | C | H | O a | N | S | |
| Rice husk | 9.45 | 71.94 | 4.16 | 14.45 | 41.28 | 5.12 | 53.11 | 0.42 | 0.07 |
| Samples | Water Content (wt%) | pH |
|---|---|---|
| AP-HT | 98.10 | 3.24 |
| AP-Pyro | 58.66 | 2.70 |
| Compounds | Molecular Formula | Relative Content (%) in | |
|---|---|---|---|
| AP-HT | AP-Pyro | ||
| Acids | |||
| Formic acid | CH2O2 | 0.136 | 2.557 |
| Acetic acid | C2H4O2 | 0.399 | 20.939 |
| Propanoic acid | C3H6O2 | 0.000 | 1.850 |
| n-Hexadecanoic acid | C16H32O2 | 2.392 | 0.000 |
| Octadecanoic acid | C18H36O2 | 2.476 | 0.000 |
| Total | 5.402 | 25.346 | |
| Furans | |||
| Furfural | C5H4O2 | 1.217 | 2.835 |
| Benzofuran, 2,3-dihydro- | C8H8O | 0.000 | 1.461 |
| 2(5H)-Furanone | C4H4O2 | 1.285 | 1.143 |
| 5-Hydroxymethylfurfural | C6H6O3 | 3.348 | 0.000 |
| 2-Furancarboxaldehyde, 5-methyl- | C6H6O2 | 0.169 | 0.000 |
| 2,4(3H,5H)-Furandione, 3-methyl- | C5H6O3 | 0.361 | 0.000 |
| 2-Furanmethanol | C5H6O2 | 0.282 | 3.261 |
| 4-Methyl-5H-furan-2-one | C5H6O2 | 0.371 | 0.000 |
| Total | 7.034 | 8.700 | |
| Ketones | |||
| 2,3-Butanedione | C4H6O2 | 0.000 | 1.162 |
| 1-Hydroxy-2-butanone | C4H8O2 | 0.000 | 2.309 |
| 2-Propanone, 1-hydroxy- | C3H6O2 | 1.825 | 10.119 |
| 2-Butanone | C4H8O | 2.720 | 0.959 |
| 2-Propanone,1-(acetyloxy)- | C5H8O3 | 0.000 | 1.544 |
| 2-Cyclopenten-1-one | C5H6O | 0.000 | 1.048 |
| 1,2-Cyclopentanedione | C8H12O2 | 1.375 | 0.000 |
| Dihydroxyacetone | C3H6O3 | 0.602 | 0.000 |
| 3-Pentanone | C5H10O | 0.437 | 0.532 |
| Apocynin | C9H10O3 | 0.579 | 0.000 |
| 2-Cyclopenten-1-one, 2-hydroxy- | C5H6O2 | 0.000 | 3.066 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | C6H8O2 | 0.567 | 2.364 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | C6H8O2 | 0.742 | 0.000 |
| 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | C10H12O3 | 0.000 | 1.004 |
| 1-Hydroxy-2-pentanone | C5H10O2 | 0.406 | 0.000 |
| 3-Pyrazolidinone, 1,4-dimethyl | C5H10N2O | 1.071 | 0.000 |
| 3,6-Nonadecadione | C19H36O2 | 1.101 | 0.000 |
| 3′,5′-Dimethoxyacetophenone | C10H12O3 | 0.283 | 0.000 |
| Total | 11.710 | 24.107 | |
| Phenol | |||
| Phenol, 2-methoxy- | C7H8O2 | 2.276 | 2.967 |
| p-Cresol | C7H8O | 0.462 | 0.000 |
| Creosol | C8H10O2 | 2.860 | 1.919 |
| Phenol | C6H6O | 0.420 | 1.189 |
| Phenol, 4-ethyl-2-methoxy- | C9H12O2 | 0.556 | 0.671 |
| 2-Methoxy-4-vinylphenol | C9H10O2 | 3.038 | 1.044 |
| trans-Isoeugenol | C10H12O2 | 0.644 | 0.000 |
| Phenol, 2,6-dimethoxy- | C8H10O3 | 1.385 | 3.191 |
| Phenol, 4-methoxy-3-(methoxymethyl)- | C9H12O3 | 0.589 | 0.000 |
| Phenol, 2,6-dimethoxy-4-(2-propenyl)- | C11H14O3 | 0.258 | 0.000 |
| Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | C10H12O4 | 0.528 | 0.000 |
| Total | 13.016 | 10.982 | |
| Sugars | |||
| d-Mannose | C6H12O6 | 0.771 | 0.000 |
| 2,3-Anhydro-d-mannosan | C6H8O4 | 0.000 | 0.807 |
| 1,43,6-Dianhydro-α-d-glucopyranose | C6H8O4 | 0.394 | 1.551 |
| d-Glycero-d-galacto-heptose | C7H14O7 | 0.000 | 0.945 |
| β-D-Glucopyranose, 1,6-anhydro- | C6H10O5 | 17.818 | 6.329 |
| Total | 18.983 | 9.632 | |
| Item | RH | RH-HT | RH-Pyro | RH-HCl |
|---|---|---|---|---|
| O/C | 1.57 | 1.33 | 1.30 | 1.17 |
| H/C | 0.11 | 0.12 | 0.12 | 0.11 |
| Samples | m1 (wt.%) | DTGmax (wt.%/min) | Tmax (°C) | Ti (°C) |
|---|---|---|---|---|
| RH | 61.8 | 6.1 | 339.5 | 270.2 |
| RH-HT | 67.2 | 7.6 | 355.8 | 294.9 |
| RH-Pyro | 64.9 | 7.8 | 358.5 | 279.6 |
| RH-HCl | 66.1 | 9.1 | 347.3 | 301.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Li, H.; Karnowo; Song, B.; Zhang, S. Integrated Leaching and Thermochemical Technologies for Producing High-Value Products from Rice Husk: Leaching of Rice Husk with the Aqueous Phases of Bioliquids. Energies 2020, 13, 6033. https://doi.org/10.3390/en13226033
Gao W, Li H, Karnowo, Song B, Zhang S. Integrated Leaching and Thermochemical Technologies for Producing High-Value Products from Rice Husk: Leaching of Rice Husk with the Aqueous Phases of Bioliquids. Energies. 2020; 13(22):6033. https://doi.org/10.3390/en13226033
Chicago/Turabian StyleGao, Wenran, Hui Li, Karnowo, Bing Song, and Shu Zhang. 2020. "Integrated Leaching and Thermochemical Technologies for Producing High-Value Products from Rice Husk: Leaching of Rice Husk with the Aqueous Phases of Bioliquids" Energies 13, no. 22: 6033. https://doi.org/10.3390/en13226033
APA StyleGao, W., Li, H., Karnowo, Song, B., & Zhang, S. (2020). Integrated Leaching and Thermochemical Technologies for Producing High-Value Products from Rice Husk: Leaching of Rice Husk with the Aqueous Phases of Bioliquids. Energies, 13(22), 6033. https://doi.org/10.3390/en13226033