Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation
Abstract
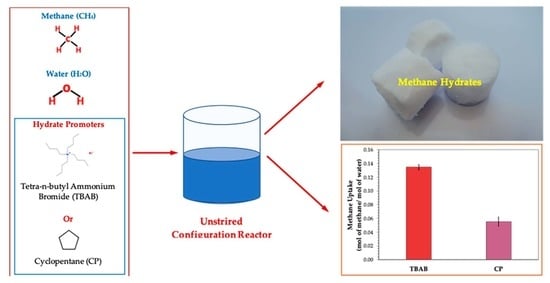
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus for the Hydrate Formation and Dissociation Experiments
2.3. Hydrate Formation
2.4. Hydrate Dissociation
3. Results and Discussion
3.1. Effects of Tetra-N-Butyl Ammonium Bromide (TBAB)
3.2. Effects of Cyclopentane (CP)
3.3. Hydrate Dissociation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IGU. Global Gas Report 2018; International Gas Union: Washington, DC, USA, 2018; p. 60. [Google Scholar]
- EIA. International Energy Outlook 2019 with Projections to 2050; U.S. Energy Information Administration, U.S. Department of Energy: Washington, DC, USA, 2019; p. 170.
- Demirbas, A. Methane hydrates as potential energy resource: Part 1–Importance, resource and recovery facilities. Energy Convers. Manag. 2010, 51, 1547–1561. [Google Scholar] [CrossRef]
- Englezos, P.; Lee, J.D. Gas hydrates: A cleaner source of energy and opportunity for innovative technologies. Korean J. Chem. Eng. 2005, 22, 671–681. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural gas hydrates–A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Zarenezhad, B.; Varaminian, F. A unified approach for description of gas hydrate formation kinetics in the presence of kinetic promoters in gas hydrate converters. Energy Convers. Manag. 2013, 73, 144–149. [Google Scholar] [CrossRef]
- Mech, D.; Gupta, P.; Sangwai, J.S. Kinetics of methane hydrate formation in an aqueous solution of thermodynamic promoters (THF and TBAB) with and without kinetic promoter (SDS). J. Nat. Gas Sci. Eng. 2016, 35, 1519–1534. [Google Scholar] [CrossRef]
- Verrett, J.; Posteraro, D.; Servio, P. Surfactant effects on methane solubility and mole fraction during hydrate growth. Chem. Eng. Sci. 2012, 84, 80–84. [Google Scholar] [CrossRef]
- Hao, W.; Wang, J.; Fan, S.; Hao, W. Evaluation and analysis method for natural gas hydrate storage and transportation processes. Energy Convers. Manag. 2008, 49, 2546–2553. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Kumar, A. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 216, 262–285. [Google Scholar] [CrossRef]
- Sangwai, J.S.; Oellrich, L. Phase equilibrium of semiclathrate hydrates of methane in aqueous solutions of tetra-n-butyl ammonium bromide (TBAB) and TBAB–NaCl. Fluid Phase Equilibria 2014, 367, 95–102. [Google Scholar] [CrossRef]
- Li, D.; Du, J.-W.; Fan, S.-S.; Liang, D.-Q.; Li, X.-S.; Huang, N.-S. Clathrate Dissociation Conditions for Methane + Tetra-n-butyl Ammonium Bromide (TBAB) + Water. J. Chem. Eng. Data 2007, 52, 1916–1918. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Eslamimanesh, A.; Belandria, V.; Richon, D. Phase Equilibria of Semiclathrate Hydrates of CO2, N2, CH4, or H2 + Tetra-n-butylammonium Bromide Aqueous Solution. J. Chem. Eng. Data 2011, 56, 3855–3865. [Google Scholar] [CrossRef]
- Li, X.-S.; Xia, Z.-M.; Chen, Z.; Yan, K.-F.; Li, G.; Wu, H.-J. Gas Hydrate Formation Process for Capture of Carbon Dioxide from Fuel Gas Mixture. Ind. Eng. Chem. Res. 2010, 49, 11614–11619. [Google Scholar] [CrossRef]
- Duc, N.H.; Chauvy, F.; Herri, J.-M. CO2 capture by hydrate crystallization–A potential solution for gas emission of steelmaking industry. Energy Convers. Manag. 2007, 48, 1313–1322. [Google Scholar] [CrossRef]
- Fan, S.; Li, S.; Wang, J.; Lang, X.; Wang, Y. Efficient Capture of CO2 from Simulated Flue Gas by Formation of TBAB or TBAF Semiclathrate Hydrates. Energy Fuels 2009, 23, 4202–4208. [Google Scholar] [CrossRef]
- Zhong, D.-L.; Englezos, P. Methane Separation from Coal Mine Methane Gas by Tetra-n-butyl Ammonium Bromide Semiclathrate Hydrate Formation. Energy Fuels 2012, 26, 2098–2106. [Google Scholar] [CrossRef]
- Zheng, J.-N.; Yang, M.; Liu, Y.; Wang, D.; Song, Y.-C. Effects of cyclopentane on CO2 hydrate formation and dissociation as a co-guest molecule for desalination. J. Chem. Thermodyn. 2017, 104, 9–15. [Google Scholar] [CrossRef]
- Lim, Y.-A.; Babu, P.; Kumar, R.; Linga, P. Morphology of Carbon Dioxide–Hydrogen–Cyclopentane Hydrates with or without Sodium Dodecyl Sulfate. Cryst. Growth Des. 2013, 13, 2047–2059. [Google Scholar] [CrossRef]
- Li, X.-S.; Cai, J.; Chen, Z.; Xu, C.-G. Hydrate-Based Methane Separation from the Drainage Coal-Bed Methane with Tetrahydrofuran Solution in the Presence of Sodium Dodecyl Sulfate. Energy Fuels 2012, 26, 1144–1151. [Google Scholar] [CrossRef]
- Siangsai, A.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S.; Linga, P. Investigation on the roles of activated carbon particle sizes on methane hydrate formation and dissociation. Chem. Eng. Sci. 2015, 126, 383–389. [Google Scholar] [CrossRef]
- Siangsai, A.; Inkong, K.; Kulprathipanja, S.; Kitiyanan, B.; Rangsunvigit, P. Roles of Sodium Dodecyl Sulfate on Tetrahydrofuran-Assisted Methane Hydrate Formation. J. Oleo Sci. 2018, 67, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Inkong, K.; Rangsunvigit, P.; Kulprathipanja, S.; Linga, P. Effects of temperature and pressure on the methane hydrate formation with the presence of tetrahydrofuran (THF) as a promoter in an unstirred tank reactor. Fuel 2019, 255, 115705. [Google Scholar] [CrossRef]
- Inkong, K.; Veluswamy, H.P.; Rangsunvigit, P.; Kulprathipanja, S.; Linga, P. Investigation on the kinetics of methane hydrate formation in the presence of methyl ester sulfonate. J. Nat. Gas Sci. Eng. 2019, 71, 102999. [Google Scholar] [CrossRef]
- Fandiño, O.; Ruffine, L. Methane hydrate nucleation and growth from the bulk phase: Further insights into their mechanisms. Fuel 2014, 117, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Ohmura, R.; Matsuda, S.; Uchida, T.; Ebinuma, T.; Narita, H. Clathrate Hydrate Crystal Growth in Liquid Water Saturated with a Guest Substance: Observations in a Methane + Water System. Cryst. Growth Des. 2005, 5, 953–957. [Google Scholar] [CrossRef]
- Arjmandi, M.; Chapoy, A.A.; Tohidi, B. Equilibrium Data of Hydrogen, Methane, Nitrogen, Carbon Dioxide, and Natural Gas in Semi-Clathrate Hydrates of Tetrabutyl Ammonium Bromide. J. Chem. Eng. Data 2007, 52, 2153–2158. [Google Scholar] [CrossRef]
- Linga, P.; Haligva, C.; Nam, S.C.; Ripmeester, J.A.; Englezos, P. Recovery of Methane from Hydrate Formed in a Variable Volume Bed of Silica Sand Particles. Energy Fuels 2009, 23, 5508–5516. [Google Scholar] [CrossRef]
- Du, J.; Li, H.; Wang, L. Effects of ionic surfactants on methane hydrate formation kinetics in a static system. Adv. Powder Technol. 2014, 25, 1227–1233. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, S.; Lee, J.W. Does SDS micellize under methane hydrate-forming conditions below the normal Krafft point? J. Colloid Interface Sci. 2007, 315, 313–318. [Google Scholar] [CrossRef]
- Ho, L.C.; Babu, P.; Kumar, R.; Linga, P. HBGS (hydrate based gas separation) process for carbon dioxide capture employing an unstirred reactor with cyclopentane. Energy 2013, 63, 252–259. [Google Scholar] [CrossRef]
- Lv, Q.; Li, L.; Li, X.-S.; Chen, Z. Formation Kinetics of Cyclopentane + Methane Hydrates in Brine Water Systems and Raman Spectroscopic Analysis. Energy Fuels 2015, 29, 6104–6110. [Google Scholar] [CrossRef]
- Yaws, C.L.; Richmond, P.C. Chapter 21-Surface tension—Organic compounds. In Thermophysical Properties of Chemicals and Hydrocarbons; Yaws, C.L., Ed.; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 686–781. [Google Scholar]
- Veluswamy, H.P.; Chen, J.Y.; Linga, P. Surfactant effect on the kinetics of mixed hydrogen/propane hydrate formation for hydrogen storage as clathrates. Chem. Eng. Sci. 2015, 126, 488–499. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Chin, W.I.; Linga, P. Clathrate hydrates for hydrogen storage: The impact of tetrahydrofuran, tetra-n-butylammonium bromide and cyclopentane as promoters on the macroscopic kinetics. Int. J. Hydrogen Energy 2014, 39, 16234–16243. [Google Scholar] [CrossRef]









| Exp. No. | TBAB(Mass Fraction) | Induction Time *(h) | Methane Uptake(mol of Methane/mol of H2O) | %Recovery |
|---|---|---|---|---|
| 1 | 11 | 0.0514 | 90.69 | |
| 2 | w = 0.025 | 15 | 0.0956 | 92.89 |
| 3 | 8 | 0.0882 | 91.28 | |
| Avg | 11.33 ± 2.87 | 0.0784 ± 0.0193 | 91.62 ± 0.93 | |
| 4 | 6.5 | 0.1298 | 90.17 | |
| 5 | w = 0.05 | 7 | 0.1352 | 88.25 |
| 6 | 6.9 | 0.1386 | 90.53 | |
| Avg | 6.80 ± 0.22 | 0.1345 ± 0.0036 | 89.65 ± 1.00 | |
| 7 | 0.8 | 0.0559 | 89.38 | |
| 8 | w = 0.10 | 1 | 0.0702 | 94.39 |
| 9 | 1 | 0.0658 | 94.55 | |
| Avg | 0.93 ± 0.09 | 0.0639 ± 0.0060 | 92.77 ± 2.40 | |
| 10 | 0.2 | 0.0095 | 90.67 | |
| 11 | w = 0.20 | 0.3 | 0.0120 | 88.95 |
| 12 | 0.36 | 0.0097 | 90.93 | |
| Avg | 0.29 ± 0.06 | 0.0104 ± 0.0011 | 90.18 ± 0.88 | |
| Exp. No. | CP (%v/v) | Induction Time * (min) | Methane Uptake (mol of Methane/mol of H2O) | %Recovery |
|---|---|---|---|---|
| 13 | 5.00 | 0.0216 | 80.59 | |
| 14 | 5 | 15.00 | 0.0242 | 82.67 |
| 15 | 4.80 | 0.0279 | 81.98 | |
| Avg | 8.27 ± 4.76 | 0.0246 ± 0.0025 | 89.65 ± 1.00 | |
| 16 | 4.80 | 0.0446 | 80.35 | |
| 17 | 10 | 9.60 | 0.0413 | 78.58 |
| 18 | 9.00 | 0.0392 | 80.90 | |
| Avg | 7.13 ± 2.13 | 0.0417 ± 0.0022 | 79.94 ± 0.99 | |
| 19 | 170.00 | 0.0628 | 79.61 | |
| 20 | 15 | 300.00 | 0.0472 | 81.41 |
| 21 | 120.00 | 0.0557 | 80.45 | |
| Avg | 196.68 ± 75.86 | 0.0552 ± 0.0063 | 80.49 ± 0.73 | |
| 22 | 126.00 | 0.0438 | 80.67 | |
| 23 | 20 | 100.02 | 0.0395 | 78.75 |
| 24 | 210.00 | 0.0386 | 80.93 | |
| Avg | 145.34 ± 46.93 | 0.0406 ± 0.0023 | 80.12 ± 0.97 | |
| Exp. No. | Experimental Temperature (°C) | Induction Time (min) | Methane Uptake (mol of Methane/mol of H2O) |
|---|---|---|---|
| 16 | 170.00 | 0.0628 | |
| 17 | 2.5 | 300.00 | 0.0472 |
| 18 | 120.00 | 0.0557 | |
| Avg. | 196.67 ± 75.86 | 0.0417 ± 0.0027 | |
| 25 | 110.20 | 0.0367 | |
| 26 | 4 | 109.00 | 0.0336 |
| 27 | 110.80 | 0.0344 | |
| Avg. | 110.00 ± 0.92 | 0.0349 ± 0.0016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanakro, W.; Jaikwang, C.; Inkong, K.; Kulprathipanja, S.; Rangsunvigit, P. Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation. Energies 2020, 13, 6518. https://doi.org/10.3390/en13246518
Chanakro W, Jaikwang C, Inkong K, Kulprathipanja S, Rangsunvigit P. Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation. Energies. 2020; 13(24):6518. https://doi.org/10.3390/en13246518
Chicago/Turabian StyleChanakro, Warintip, Chutikan Jaikwang, Katipot Inkong, Santi Kulprathipanja, and Pramoch Rangsunvigit. 2020. "Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation" Energies 13, no. 24: 6518. https://doi.org/10.3390/en13246518
APA StyleChanakro, W., Jaikwang, C., Inkong, K., Kulprathipanja, S., & Rangsunvigit, P. (2020). Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation. Energies, 13(24), 6518. https://doi.org/10.3390/en13246518