Synthesis and Characterization of Novel Green Hybrid Nanocomposites for Application as Proton Exchange Membranes in Direct Borohydride Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PO4-TiO2 and SO4-TiO2 Nanotubes Doping Agents
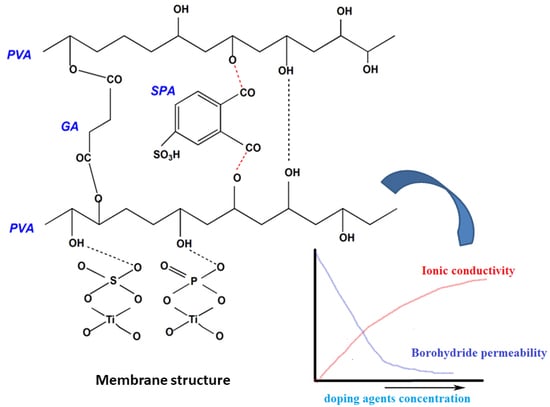
2.3. Preparation of SPVA-SPTiO Blend Membranes
2.4. Characterization of Doping Agents and SPTiO-Polymer Composite Membranes
2.4.1. Structural Analysis and Morphology
2.4.2. Thermal and Mechanical Analysis
2.4.3. Swelling Ratio, Water Uptake and Contact Angle
2.4.4. Oxidative Stability
2.4.5. IEC, Ionic Conductivity and Borohydride Permeability
3. Results and Discussion
3.1. Characterization of PO4-TiO2 and SO4-TiO2 Doping Agents
3.2. Characterization of Membranes
3.2.1. Structural Analysis and Morphology
3.2.2. Thermal and Mechanical Analysis
3.2.3. Swelling Ratio, Water Uptake and Contact Angle
3.2.4. Oxidative Stability
3.2.5. IEC and Borohydride Permeability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merino-Jiménez, I.; León, C.P.; Shah, A.A.; Walsh, F.C. Developments in direct borohydride fuel cells and remaining challenges. J. Power Sources 2012, 219, 339–357. [Google Scholar] [CrossRef]
- Ma, J.; Choudhury, N.A.; Sahai, Y. A comprehensive review of direct borohydride fuel cells. Renew. Sustain. Energy Rev. 2010, 14, 183–199. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Liquid fuel cells. Beilstein J. Nanotechnol. 2014, 5, 1399–1418. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.F.; Sequeira, C.A.C. Sodium borohydride as a fuel for the future. Renew. Sustain. Energy Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Stroman, R.O.; Jackson, G.S. Modeling the performance of an ideal NaBH4-H2O2 direct borohydride fuel cell. Int. J. Hydrogen Energy 2014, 247, 756–769. [Google Scholar]
- Santos, D.M.F.; Sequeira, C.A.C. Effect of membrane separators on the performance of direct borohydride fuel cells. J. Electrochem. Soc. 2012, 159, B126–B132. [Google Scholar] [CrossRef]
- Šljukić, B.; Morais, A.L.; Santos, D.M.F.; Sequeira, C.A.C. Anion- or cation exchange membranes for NaBH4/H2O2 fuel cells? Membranes 2012, 2, 478–492. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Water soluble polymers as proton exchange membranes for fuel cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef] [Green Version]
- Pourzare, K.; Mansourpanah, Y.; Farhadi, S. Advanced nanocomposite membranes for fuel cell applications: A comprehensive review. Biofuel Res. J. 2016, 12, 496–513. [Google Scholar] [CrossRef] [Green Version]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Abdullah, N.; Kamarudin, S.K. Titanium dioxide in fuel cell technology: An overview. J. Power Sources 2015, 278, 109–118. [Google Scholar] [CrossRef]
- Bet-moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Matos, B.R.; Isidoro, R.A.; Santiago, E.I.; Tavares, A.C.; Ferlauto, A.S.; Muccillo, R.; Fonseca, F.C. Nafion-titanate nanotubes composites prepared by in situ crystallization and casting for direct ethanol fuel cells. Int. J. Hydrogen Energy 2015, 40, 1859–1867. [Google Scholar] [CrossRef]
- Lee, C.; Park, J.; Jeon, Y.; Park, J.; Einaga, H.; Truong, Y.B.; Kyratzis, I.L.; Mochida, I.; Choi, J.; Shul, Y.G. Phosphate-modified TiO2/ZrO2 nanofibrous web composite membrane for enhanced performance and durability of high-temperature proton exchange membrane fuel cells. Energy Fuels 2017, 31, 7645–7652. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Peng, S.; Lu, G.; Li, S. Modification of TiO2 with sulfate and phosphate for enhanced eosin Y-sensitized hydrogen evolution under visible light illumination. Photochem. Photobiol. Sci. 2013, 12, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, Y.; Endo, M. Ion-conductive properties of polyether-based composite electrolytes filled with mesoporous silica, alumina and titania. Electrochim. Acta 2013, 113, 361–365. [Google Scholar] [CrossRef]
- Devrim, Y.; Erkan, S.; Bac, N.; Eroglu, I. Preparation and characterization of sulfonated polysulfone/titanium dioxide composite membranes for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 3467–3475. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Javanbakht, M.; Pourmahdian, S. Enhancing the performance of SPEEK polymer electrolyte membranes using functionalized TiO2 nanoparticles with proton hopping sites. RSC Adv. 2017, 7, 8303–8313. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Chien, W.; Li, Y.J. Direct methanol fuel cell based on poly(vinyl alcohol)/titanium oxide nanotubes/poly(styrene sulfonic acid) (PVA/nt-TiO2/PSSA) composite polymer membrane. J. Power Sources 2010, 195, 3407–3415. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.; Wang, Y.; Chai, W.; Yang, M. Measurement and modeling of the effect of composition ratios on the properties of poly(vinyl alcohol)/poly(vinyl pyrrolidone) membranes. Mater. Des. 2016, 103, 249–258. [Google Scholar] [CrossRef]
- Herring, A.M. Inorganic–polymer composite membranes for proton exchange membrane fuel cells. J. Macromol. Sci. Polymer Rev. 2006, 46, 245–296. [Google Scholar] [CrossRef]
- Onoda, H.; Matsukura, A. Influence of concentration in phosphoric acid treatment of titanium oxide and their powder properties. J. Asian Ceram. Soc. 2015, 3, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Benito, H.E.; Sánchez, T.; Alamilla, R.G.; Enríquez, J.M.; Robles, G.S.; Delgado, F.P. Synthesis and physicochemical characterization of titanium oxide and sulfated titanium oxide obtained by thermal hydrolysis of titanium tetrachloride. Braz. J. Chem. Eng. 2014, 31, 737–745. [Google Scholar] [CrossRef]
- Lin, C.; Chien, S.; Chao, J.; Sheu, C.; Cheng, Y.; Huang, Y.; Tsai, C. The synthesis of sulfated titanium oxide nanotubes. Catal. Lett. 2002, 80, 153–159. [Google Scholar] [CrossRef]
- Gouda, M.H.; Gouveia, W.; Afonso, M.L.; Šljukić, B.; El Essawy, N.A.; Nassr, A.B.A.A.; Santos, D.M.F. Poly(vinyl alcohol)-based crosslinked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J. Power Sources 2019, 432, 92–101. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Farag, H.A.; Tamer, T.M.; Konsowa, A.H.; Gouda, M.H. Development of novel iota carrageenan-g-polyvinyl alcohol polyelectrolyte membranes for direct methanol fuel cell application. Polym. Bull. 2019. [Google Scholar] [CrossRef]
- Kumar, K.S.; Rajendran, S.; Prabhu, M.R. A study of influence on sulfonated TiO2-poly (vinylidene fluoride-co-hexafluoropropylene) nano composite membranes for PEM fuel cell application. Appl. Surf. Sci. 2017, 418, 64–71. [Google Scholar] [CrossRef]
- Lu, M.; Wang, F.; Liao, Q.; Chen, K.; Qin, J.; Pan, S. FTIR spectra and thermal properties of TiO2-doped iron phosphate glasses. J. Mol. Struct. 2015, 1081, 187–192. [Google Scholar] [CrossRef]
- Goswami, P.; Ganguli, J. Synthesis characterization and photocatalytic reactions of phosphated mesoporous titania. Bull. Mater. Sci. 2012, 35, 889–896. [Google Scholar] [CrossRef]
- Venkatesan, P.; Dharmalingam, S. Effect of cation transport of SPEEK-Rutile TiO2 electrolyte on microbial fuel cell performance. J. Membr. Sci. 2015, 492, 518–527. [Google Scholar] [CrossRef]
- Jun, Y.; Zarrin, H.; Fowler, M.; Chen, Z. Functionalized titania nanotube composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6073–6081. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chuang, L.C.; Kannan, A.M.; Lin, C.W. Proton-conducting membranes with high selectivity from cross-linked poly(vinyl alcohol) and poly(vinyl pyrrolidone) for direct methanol fuel cell applications. J. Power Sources 2009, 186, 22–28. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Lin, R.; Mac, J.; Liu, J. Alkaline solid polymer electrolyte membranes based on structurally modified PVA/PVP with improved alkali stability. Polymer 2010, 51, 4850–4859. [Google Scholar] [CrossRef]
- Rochliadi, A.; Bundjali, B.; Arcana, I.M. Polymer electrolyte membranes prepared by blending of poly(vinyl alcohol)-poly(ethylene oxide) for lithium battery application. In Proceedings of the Joint International Conference on Electric Vehicular Technology and Industrial, Mechanical, Electrical and Chemical Engineering (ICEVT & IMECE), Surakarta, Indonesia, 4–5 November 2015; pp. 370–373. [Google Scholar]
- Ozdemir, Y.; Uregen, N.; Devrim, Y. Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2648–2657. [Google Scholar] [CrossRef]
- Li, T.; Zhong, G.; Fu, R.; Yang, Y. Synthesis and characterization of Nafion/cross-linked PVP semi-interpenetrating polymer network membrane for direct methanol fuel cell. J. Membr. Sci. 2010, 354, 189–197. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Moghaddam, J.A. Investigation of physicochemical and electrochemical properties of recast Nafion nanocomposite membranes using different loading of zirconia nanoparticles for proton exchange membrane fuel cell applications. Mater. Sci. Energy Technol. 2018, 1, 146–154. [Google Scholar] [CrossRef]
- An, L.; Jung, C.Y. Transport phenomena in direct borohydride fuel cells. Appl. Energy 2017, 205, 1270–1282. [Google Scholar] [CrossRef]











| Sample | Ti EDX wt.% | O EDX wt.% | S EDX/SICP wt.% | P EDX/P ICP wt.% |
|---|---|---|---|---|
| SO4-TiO2 | 41.7 | 52.8 | 5.5/4.9 | − |
| PO4-TiO2 | 40.3 | 54.0 | − | 5.7/5.3 |
| Membrane | Thickness/µm | Water uptake/% | Swelling ratio/% | Contact angle/° | Tensile strength/MPa |
|---|---|---|---|---|---|
| SPVA | 110 | 110 | 90 | 75 | 4.5 |
| SPVA-SPTiO-1 | 140 | 60 | 28 | 77 | 11.5 |
| SPVA-SPTiO-2 | 145 | 30 | 17 | 78 | 20.3 |
| SPVA-SPTiO-3 | 150 | 13 | 7 | 80 | 28.2 |
| Nafion®117 | 183 | 15 | 8 | 102 | 25 |
| Membrane | Oxidative stability/RW, %* | IEC/meq g−1 | Borohydride permeability/cm2 s−1 | Ionic Conductivity /mS cm−1 |
|---|---|---|---|---|
| SPVA | 80 | 0.10 | 0.71 × 10−5 | 1.25 |
| SPVA-SPTiO-1 | 90 | 0.25 | 0.49 × 10−6 | 3.12 |
| SPVA-SPTiO-2 | 99.5 | 0.40 | 0.39 × 10−6 | 5.57 |
| SPVA-SPTiO-3 | 98 | 0.50 | 0.32 × 10−6 | 7.13 |
| Nafion®117 | 92 | 0.89 | 0.40 × 10−6 | 45.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, M.H.; Elessawy, N.A.; Santos, D.M.F. Synthesis and Characterization of Novel Green Hybrid Nanocomposites for Application as Proton Exchange Membranes in Direct Borohydride Fuel Cells. Energies 2020, 13, 1180. https://doi.org/10.3390/en13051180
Gouda MH, Elessawy NA, Santos DMF. Synthesis and Characterization of Novel Green Hybrid Nanocomposites for Application as Proton Exchange Membranes in Direct Borohydride Fuel Cells. Energies. 2020; 13(5):1180. https://doi.org/10.3390/en13051180
Chicago/Turabian StyleGouda, Marwa H., Noha A. Elessawy, and Diogo M.F. Santos. 2020. "Synthesis and Characterization of Novel Green Hybrid Nanocomposites for Application as Proton Exchange Membranes in Direct Borohydride Fuel Cells" Energies 13, no. 5: 1180. https://doi.org/10.3390/en13051180
APA StyleGouda, M. H., Elessawy, N. A., & Santos, D. M. F. (2020). Synthesis and Characterization of Novel Green Hybrid Nanocomposites for Application as Proton Exchange Membranes in Direct Borohydride Fuel Cells. Energies, 13(5), 1180. https://doi.org/10.3390/en13051180