Spherical Sb Core/Nb2O5-C Double-Shell Structured Composite as an Anode Material for Li Secondary Batteries
Abstract
:1. Introduction
2. Experimental Section
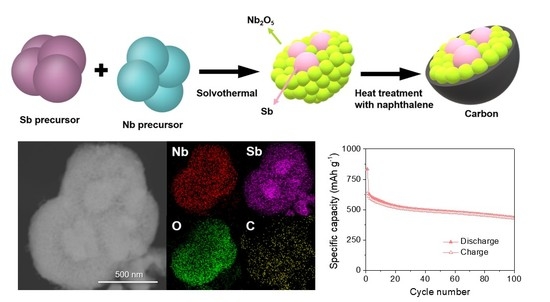
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-ion battery storage for the grid—A review of stationary battery storage system design tailored for applications in modern power grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef] [Green Version]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- De Sutter, L.; Berckmans, G.; Marinaro, M.; Smekens, J.; Firouz, Y.; Wohlfahrt-Mehrens, M.; Van Mierlo, J.; Omar, N. Comprehensive aging analysis of volumetric constrained lithium-ion pouch cells with high concentration silicon-alloy anodes. Energies 2018, 11, 2948. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Lv, H.; Qiu, S.; Lu, G.; Fu, Y.; Li, X.; Hu, C.; Liu, J. Nanostructured Antimony/carbon Composite Fibers as Anode Material for Lithium-ion Battery. Electrochim. Acta 2015, 151, 214–221. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, F.; Chen, Z.; He, X.; Li, Q.; Wang, H. Metallic Sb nanoparticles embedded in carbon nanosheets as anode material for lithium ion batteries with superior rate capability and long cycling stability. Electrochim. Acta 2018, 283, 1689–1694. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Q.; Zheng, F.; Ou, X.; Yang, C.; Xiong, X.; Liu, M.; Hu, D.; Huang, C. Sb@C/expanded graphite as high-performance anode material for lithium ion batteries. J. Alloys Compd. 2018, 744, 481–486. [Google Scholar] [CrossRef]
- Yoon, S.; Manthiram, A. Sb-MOx-C (M = Al, Ti, or Mo) Nanocomposite Anodes for Lithium-Ion Batteries. Chem. Mater. 2009, 21, 3898–3904. [Google Scholar] [CrossRef]
- Park, C.-M.; Sohn, H.-J. Electrochemical Characteristics of TiSb2 and Sb/TiC/C Nanocomposites as Anodes for Rechargeable Li-Ion Batteries. J. Electrochem. Soc. 2010, 157, A46–A49. [Google Scholar] [CrossRef]
- Sung, J.H.; Park, C.-M. Amorphized Sb-based composite for high-performance Li-ion battery anodes. J. Electroanal. Chem. 2013, 700, 12–16. [Google Scholar] [CrossRef]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. One-Dimensional Yolk–Shell Sb@Ti–O–P Nanostructures as a High-Capacity and High-Rate Anode Material for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 447–454. [Google Scholar] [CrossRef]
- Seo, H.; Kim, H.-S.; Kim, K.; Choi, H.; Kim, J.-H. Magnesium silicide-derived porous Sb-Si-C composite for stable lithium storage. J. Alloys Compd. 2019, 782, 525–532. [Google Scholar] [CrossRef]
- Dailly, A.; Schneider, R.; Billaud, D.; Fort, Y.; Willmann, P. New graphite–antimony composites as anodic materials for lithium-ion batteries.: Preparation, characterisation and electrochemical performance. Electrochim. Acta 2002, 47, 4207–4212. [Google Scholar] [CrossRef]
- Dailly, A.; Ghanbaja, J.; Willmann, P.; Billaud, D. Lithium insertion into new graphite–antimony composites. Electrochim. Acta 2003, 48, 977–984. [Google Scholar] [CrossRef]
- Fuchsbichler, B.; Stangl, C.; Kren, H.; Uhlig, F.; Koller, S. High capacity graphite–silicon composite anode material for lithium-ion batteries. J. Power Sources 2011, 196, 2889–2892. [Google Scholar] [CrossRef]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. Double-Walled Sb@TiO2−x Nanotubes as a Superior High-Rate and Ultralong-Lifespan Anode Material for Na-Ion and Li-Ion Batteries. Adv. Mater. 2016, 28, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ji, C.; Pan, Q.; Zhang, X.; Zhang, J.; Wang, H.; Liao, T.; Li, Q. Scalable synthesis of Sb/MoS2/C composite as high performance anode material for lithium ion batteries. J. Alloys Compd. 2017, 728, 1139–1145. [Google Scholar] [CrossRef]
- Choi, H.; Kim, K.; Cho, W.; Park, C.-M.; Kim, J.-H. Synthesis and electrochemical reaction mechanism of Zn-TiOx-C nanocomposite anode materials for Li secondary batteries. J. Electrochem. Soc. 2017, 164, A2683–A2688. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Chen, Z.; Augustyn, V.; Ma, X.; Wang, G.; Dunn, B.; Lu, Y. High-Performance Supercapacitors Based on Nanocomposites of Nb2O5 Nanocrystals and Carbon Nanotubes. Adv. Energy Mater. 2011, 1, 1089–1093. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Free-Standing T-Nb2O5/Graphene Composite Papers with Ultrahigh Gravimetric/Volumetric Capacitance for Li-Ion Intercalation Pseudocapacitor. ACS Nano 2015, 9, 11200–11208. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.-H.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.-S.; Roh, K.C.; et al. Facile Synthesis of Nb2O5@Carbon Core–Shell Nanocrystals with Controlled Crystalline Structure for High-Power Anodes in Hybrid Supercapacitors. ACS Nano 2015, 9, 7497–7505. [Google Scholar] [CrossRef]
- Come, J.; Augustyn, V.; Kim, J.W.; Rozier, P.; Taberna, P.-L.; Gogotsi, P.; Long, J.W.; Dunn, B.; Simon, P. Electrochemical Kinetics of Nanostructured Nb2O5 Electrodes. J. Electrochem. Soc. 2014, 161, A718–A725. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, M.-S.; Cha, P.-R.; Kang, S.H.; Kim, J.-H. Structural Modification of Self-Organized Nanoporous Niobium Oxide via Hydrogen Treatment. Chem. Mater. 2016, 28, 1453–1461. [Google Scholar] [CrossRef]
- Viet, A.L.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R.; Ramakrishna, S. Nanostructured Nb2O5 Polymorphs by Electrospinning for Rechargeable Lithium Batteries. J. Phys. Chem. C 2010, 114, 664–671. [Google Scholar] [CrossRef]
- Rani, R.A.; Zoolfakar, A.S.; O’Mullane, A.P.; Austin, M.W.; Kalantar-Zadeh, K. Thin films and nanostructures of niobium pentoxide: Fundamental properties, synthesis methods and applications. J. Mater. Chem. A 2014, 2, 15683–15703. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Cao, X.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Revisiting Li+ intercalation into various crystalline phases of Nb2O5 anchored on graphene sheets as pseudocapacitive electrodes. J. Power Sources 2016, 309, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Woo, S.-G.; Jo, Y.N.; Lee, J.; Kim, J.-H. Niobium oxide nanoparticle core–amorphous carbon shell structure for fast reversible lithium storage. Electrochim. Acta 2017, 240, 316–322. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Z.; Du, L.; Li, S.; Ji, S.; Sun, J. A core–shell Si@Nb2O5 composite as an anode material for lithium-ion batteries. RSC Adv. 2016, 6, 39728–39733. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.-H. Bottom-up self-assembly of nano-netting cluster microspheres as high-performance lithium storage materials. J. Mater. Chem. A 2018, 6, 13321–13330. [Google Scholar] [CrossRef]
- Kim, K.; Seo, H.; Kim, H.-S.; Lee, H.S.; Kim, J.-H. Three-dimensional Ge/GeO2 shell-encapsulated Nb2O5 nanoparticle assemblies for high-performance lithium-ion battery anodes. Electrochim. Acta 2020, 340, 135952. [Google Scholar] [CrossRef]
- Yu, B.-C.; Hwa, Y.; Kim, J.-H.; Sohn, H.-J. Carbon coating for Si nanomaterials as high-capacity lithium battery electrodes. Electrochem. Commun. 2014, 46, 144–147. [Google Scholar] [CrossRef]
- Li, P.; Yu, L.; Ji, S.; Xu, X.; Liu, Z.; Liu, J.; Liu, J. Facile synthesis of three-dimensional porous interconnected carbon matrix embedded with Sb nanoparticles as superior anode for Na-ion batteries. Chem. Eng. J. 2019, 374, 502–510. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kalabin, I.E.; Kesler, V.G.; Pervukhina, N.V. Nb 3d and O 1s core levels and chemical bonding in niobates. J. Electron Spectrosc. 2005, 142, 129–134. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Lu, Y.; Han, X.; Cheng, F.; Chen, J. Spherical nano-Sb@C composite as a high-rate and ultra-stable anode material for sodium-ion batteries. Nano Res. 2015, 8, 3384–3393. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Kim, K.; Kim, J.-H. Spherical Sb Core/Nb2O5-C Double-Shell Structured Composite as an Anode Material for Li Secondary Batteries. Energies 2020, 13, 1999. https://doi.org/10.3390/en13081999
Seo H, Kim K, Kim J-H. Spherical Sb Core/Nb2O5-C Double-Shell Structured Composite as an Anode Material for Li Secondary Batteries. Energies. 2020; 13(8):1999. https://doi.org/10.3390/en13081999
Chicago/Turabian StyleSeo, Hyungeun, Kyungbae Kim, and Jae-Hun Kim. 2020. "Spherical Sb Core/Nb2O5-C Double-Shell Structured Composite as an Anode Material for Li Secondary Batteries" Energies 13, no. 8: 1999. https://doi.org/10.3390/en13081999
APA StyleSeo, H., Kim, K., & Kim, J. -H. (2020). Spherical Sb Core/Nb2O5-C Double-Shell Structured Composite as an Anode Material for Li Secondary Batteries. Energies, 13(8), 1999. https://doi.org/10.3390/en13081999