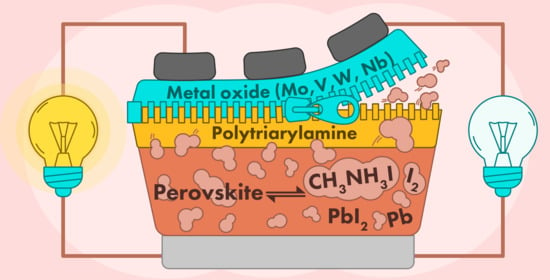
Combination of Metal Oxide and Polytriarylamine: A Design Principle to Improve the Stability of Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device and Glass Fabrication
2.1.1. ETL Deposition
2.1.2. Perovskite Deposition
2.1.3. Double-HTL Deposition and Top Electrodes
2.2. Device and Glass Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- NREL. Best Research-Cell Efficiency Chart. 2021. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 15 July 2021).
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643. [Google Scholar] [CrossRef] [Green Version]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef] [Green Version]
- Akbulatov, A.F.; Luchkin, S.Y.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the Intrinsic Thermal and Photochemical Stability of Hybrid and Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef]
- Sanehira, E.M.; Tremolet de Villers, B.J.; Schulz, P.; Reese, M.O.; Ferrere, S.; Zhu, K.; Lin, L.Y.; Berry, J.J.; Luther, J.M. Influence of Electrode Interfaces on the Stability of Perovskite Solar Cells: Reduced Degradation Using MoOx/Al for Hole Collection. ACS Energy Lett. 2016, 1, 38–45. [Google Scholar] [CrossRef]
- Kim, G.-W.; Kang, G.; Choi, K.; Choi, H.; Park, T. Solution Processable Inorganic–Organic Double-Layered Hole Transport Layer for Highly Stable Planar Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1801386. [Google Scholar] [CrossRef]
- Peng, H.; Sun, W.; Li, Y.; Ye, S.; Rao, H.; Yan, W.; Zhou, H.; Bian, Z.; Huang, C. Solution processed inorganic V2Ox as interfacial function materials for inverted planar-heterojunction perovskite solar cells with enhanced efficiency. Nano Res. 2016, 9, 2960–2971. [Google Scholar] [CrossRef]
- Haider, M.; Zhen, C.; Wu, T.; Liu, G.; Cheng, H.-M. Boosting efficiency and stability of perovskite solar cells with nickel phthalocyanine as a low-cost hole transporting layer material. J. Mater. Sci. Technol. 2018, 34, 1474–1480. [Google Scholar] [CrossRef]
- De Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke TKalantar-zadeh, K. Molybdenum Oxides—From Fundamentals to Functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Meng, B.; Zhang, B.; Xie, Z.; Wang, L. Facile Preparation of Molybdenum Bronzes as an Efficient Hole Extraction Layer in Organic Photovoltaics. ACS Appl. Mater. Interfaces 2015, 7, 13590–13596. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl TKahn, A. Transition Metal Oxides for Organic Electronics: Energetics, Device Physics and Applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef] [PubMed]
- Gerling, L.G.; Voz, C.; Alcubilla, R.; Puigdollers, J. Origin of passivation in hole-selective transition metal oxides for crystalline silicon heterojunction solar cells. J. Mater. Res. 2017, 32, 260–268. [Google Scholar] [CrossRef] [Green Version]
- You, G.; Zhuang, Q.; Wang, L.; Lin, X.; Zou, D.; Lin, Z.; Zhen, H.; Zhuang, W.; Ling, Q. Dopant-Free, Donor-Acceptor-Type Polymeric Hole-Transporting Materials for the Perovskite Solar Cells with Power Conversion Efficiencies over 20%. Adv. Energy Mater. 2020, 10, 1903146. [Google Scholar] [CrossRef]
- Tepliakova, M.M.; Mikheeva, A.N.; Frolova, L.A.; Boldyreva, A.G.; Elakshar, A.; Novikov, A.V.; Tsarev, S.A.; Ustinova, M.I.; Yamilova, O.R.; Nasibulin, A.G.; et al. Incorporation of Vanadium(V) Oxide in Hybrid Hole Transport Layer Enables Long-term Operational Stability of Perovskite Solar Cells. J. Phys. Chem. Lett. 2020, 11, 5563–5568. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, C.; Li, J.; Bai, Y.; Wang, F.; Liu, L.; Zhang, B.; Hayat, T.; Alsaedi ATan, Z.a. Low-temperature solution-processed vanadium oxide as hole transport layer for efficient and stable perovskite solar cells. Phys. Chem. Chem. Phys. 2018, 20, 21746–21754. [Google Scholar] [CrossRef] [PubMed]
- Schloemer, T.H.; Raiford, J.A.; Gehan, T.S.; Moot, T.; Nanayakkara, S.; Harvey, S.P.; Bramante, R.C.; Dunfield, S.; Louks, A.E.; Maughan, A.E.; et al. The Molybdenum Oxide Interface Limits the High-Temperature Operational Stability of Unencapsulated Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2349–2360. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo JSeok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234. [Google Scholar] [CrossRef] [PubMed]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Asokan, V.; Yuvapragasam, A.; Madurai Ramakrishnan, V.; Palanisamy, S.E.; Sundaram SVelauthapillai, D. A review on the classification of organic/inorganic/carbonaceous hole transporting materials for perovskite solar cell application. Arab. J. Chem. 2020, 13, 2526–2557. [Google Scholar] [CrossRef]
- Gao, L.; Schloemer, T.H.; Zhang, F.; Chen, X.; Xiao, C.; Zhu, K.; Sellinger, A. Carbazole-Based Hole-Transport Materials for High-Efficiency and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 4492–4498. [Google Scholar] [CrossRef]
- Yao, X.; Xu, W.; Huang, X.; Qi, J.; Yin, Q.; Jiang, X.; Huang, F.; Gong, X.; Cao, Y. Solution-processed vanadium oxide thin film as the hole extraction layer for efficient hysteresis-free perovskite hybrid solar cells. Org. Electron. 2017, 47, 85–93. [Google Scholar] [CrossRef]
- Tseng, Z.L.; Chen, L.C.; Chiang, C.H.; Chang, S.H.; Chen, C.C.; Wu, C.G. Efficient inverted-type perovskite solar cells using UV-ozone treated MoOx and WOx as hole transporting layers. Sol. Energy 2016, 139, 484–488. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Q.; Wan, L.; Zhang, W.; Fu, S.; Zhang, H.; Ling, X.; Yuan, J.; Miao, L.; Shen, C.; et al. Solution-Processed MoOx Hole-Transport Layer with F4-TCNQ Modification for Efficient and Stable Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 5862–5870. [Google Scholar] [CrossRef]
- Hou, F.; Su, Z.; Jin, F.; Yan, X.; Wang, L.; Zhao, H.; Zhu, J.; Chu, B.; Li, W. Efficient and stable planar heterojunction perovskite solar cells with an MoO3/PEDOT:PSS hole transporting layer. Nanoscale 2015, 7, 9427. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Zhang, J.; Li, X.; Cheng, C.; Tian, Y.; Jen, A.K.; Xu, B. Intensive Exposure of Functional Rings of a Polymeric Hole-Transporting Material Enables Efficient Perovskite Solar Cells. Adv. Mater. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Wang, H.; Song, X.; Liu, H.; Yang, J.; Liu, K.; Ma, H. Improving the stability of the perovskite solar cells by V2O5 modified transport layer film. RSC Adv. 2017, 7, 18456–18465. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.; Zhao, M.; Zhao, C.; Wang, Y.; Li, J.; Han, W.; Hu, Q.; Yao, L.; Jian, H.; Lu, F.; et al. Inverted CH3NH3PbI3 perovskite solar cells based on solution-processed V2O5 film combined with P3CT salt as hole transport layer. Mater. Today Energy 2018, 9, 487–495. [Google Scholar] [CrossRef]
- Raiford, J.A.; Belisle, R.A.; Bush, K.A.; Prasanna, R.; Palmstrom, A.F.; McGehee, M.D.; Bent, S.F. Atomic layer deposition of vanadium oxide to reduce parasitic absorption and improve stability in n–i–p perovskite solar cells for tandems. Sustain. Energy Fuels 2019, 3, 1517–1525. [Google Scholar] [CrossRef]
- Kim, J.; Kim, G.; Back, H.; Kong, J.; Hwang, I.-W.; Kim, T.K.; Kwon, S.; Lee, J.-H.; Lee, J.; Yu, K.; et al. High-Performance Integrated Perovskite and Organic Solar Cells with Enhanced Fill Factors and Near-Infrared Harvesting. Adv. Mater. 2016, 28, 3159–3165. [Google Scholar] [CrossRef]
- Chang, C.-C.; Tao, J.-H.; Tsai, C.-E.; Cheng, Y.-J.; Hsu, C.-S. Cross-linked Triarylamine-Based Hole-Transporting Layer for Solution-Processed PEDOT:PSS-Free Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 21466–21471. [Google Scholar] [CrossRef]
- Eze, V.O.; Seike, Y.; Mori, T. Efficient planar perovskite solar cells using solution-processed amorphous WOx/fullerene C60 as electron extraction layers. Org. Electron. 2017, 46, 253–262. [Google Scholar] [CrossRef]
- Liu, P.; Wang, C.; Zhou, D.; Yuan, Q.; Wang, Y.; Hu, Y.; Han, D.; Feng, L. Wox@Pedot Core–Shell Nanorods: Hybrid Hole-Transporting Materials for Efficient and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 1742–1752. [Google Scholar] [CrossRef]
- Yi, H.; Wang, D.; Duan, L.; Haque, F.; Xu, C.; Zhang, Y.; Conibeer, G.; Uddin, A. Solution-processed WO3 and water-free PEDOT:PSS composite for hole transport layer in conventional perovskite solar cell. Electrochim. Acta 2019, 319, 349–358. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Palilis, L.C.; Georgiadou, D.G.; Douvas, A.M.; Argitis, P.; Kennou, S.; Sygellou, L.; Papadimitropoulos, G.; Kostis, I.; Stathopoulos, N.A.; et al. Reduction of Tungsten Oxide: A Path towards Dual Functionality Utilization for Efficient Anode and Cathode Interfacial Layers in Organic Light-Emitting Diodes. Adv. Funct. Mater. 2011, 21, 1489–1497. [Google Scholar] [CrossRef]
- Tepliakova, M.; Yakushenko, I.K.; Romadina, E.I.; Novikov, A.V.; Kuznetsov, P.M.; Stevenson, K.J.; Troshin, P.A. Strength of attraction: Pyrene-based hole-transport materials with effective π–π stacking for dopant-free perovskite solar cells. Sustain. Energy Fuels 2021, 5, 283–288. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, Y.; Zhu, P.; Li, Q.; Gao, Y.; Tong, J.; Shi, L.; Zhou, Q.; Ling, C.; Chen, Q.; et al. Photo-oxidative degradation of methylammonium lead iodide perovskite: Mechanism and protection. J. Mater. Chem. A 2019, 7, 2275–2282. [Google Scholar] [CrossRef]
- Ouyang, Y.; Shi, L.; Li, Q.; Wang, J. Role of Water and Defects in Photo-Oxidative Degradation of Methylammonium Lead Iodide Perovskite. Small Methods 2019, 3, 1900154. [Google Scholar] [CrossRef]
- Hummelen, J.C.; Knight, B.W.; LePeq, F.; Wudl, F.; Yao, J.; Wilkins, C.L. Preparation and Characterization of Fulleroid and Methanofullerene Derivatives. J. Org. Chem. 1995, 60, 532–538. [Google Scholar] [CrossRef]
- Tepliakova, M.M.; Akkuratov, A.V.; Tsarev, S.A.; Troshin, P.A. Suzuki polycondensation for the synthesis of polytriarylamines: A method to improve hole-transport material performance in perovskite solar cells. Tetrahedron Lett. 2020, 61, 152317. [Google Scholar] [CrossRef]
- Tsarev, S.; Dubinina, T.S.; Luchkin, S.Y.; Zhidkov, I.S.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Phenyl-C61-butyric Acid as an Interface Passivation Layer for Highly Efficient and Stable Perovskite Solar Cells. J. Phys. Chem. C 2020, 124, 1872–1877. [Google Scholar] [CrossRef]
- Emeka, N.C.; Imoisili, P.E.; Jen, T.-C. Preparation and Characterization of NbxOy Thin Films: A Review. Coatings 2020, 10, 1246. [Google Scholar] [CrossRef]
- Tsarev, S.; Olthof, S.; Boldyreva, A.G.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P.A. Reactive modification of zinc oxide with methylammonium iodide boosts the operational stability of perovskite solar cells. Nano Energy 2021, 83, 105774. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Christians, J.A.; Schulz, P.; Tinkham, J.S.; Schloemer, T.H.; Harvey, S.P.; Tremolet de Villers, B.J.; Sellinger, A.; Berry, J.J.; Luther, J.M. Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Boldyreva, A.G.; Akbulatov, A.F.; Elnaggar, M.; Luchkin, S.Y.; Danilov, A.V.; Zhidkov, I.S.; Yamilova, O.R.; Fedotov, Y.S.; Bredikhin, S.I.; Kurmaev, E.Z.; et al. Impact of charge transport layers on the photochemical stability of MAPbI3 in thin films and perovskite solar cells. Sustain. Energy Fuels 2019, 3, 2705–2716. [Google Scholar] [CrossRef]
- Oku, T. Crystal Structures of CH3NH3PbI3 and Related Perovskite Compounds Used for Solar Cells, Solar Cells—New Approaches and Reviews; IntechOpen: London, UK, 2015. [Google Scholar]
- Harvey, S.P.; Li, Z.; Christians, J.A.; Zhu, K.; Luther, J.M.; Berry, J.J. Probing Perovskite Inhomogeneity beyond the Surface: TOF-SIMS Analysis of Halide Perovskite Photovoltaic Devices. ACS Appl. Mater. Interfaces 2018, 10, 28541–28552. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, A.; Deng, X.; Xie, L.; Xiao, A.; Li, C.; Xiang, Y.; Li, T.; Ding, L.; Hao, F. Lewis acid/base approach for efficacious defect passivation in perovskite solar cells. J. Mater. Chem. A 2020, 8, 12201–12225. [Google Scholar] [CrossRef]
- López, C.A.; Abia, C.; Rodrigues, J.E.; Serrano-Sánchez, F.; Nemes, N.M.; Martínez, J.L.; Fernandez-Díaz, M.T.; Biškup, N.; Alvarez-Galván, C.; Carrascoso, F.; et al. Enhanced Stability in CH3NH3PbI3 Hybrid Perovskite from Mechano-Chemical Synthesis: Structural, Microstructural and Optoelectronic Characterization. Sci. Rep. 2020, 10, 11228. [Google Scholar] [CrossRef]





| MOx | Thickness, nm | Voc, mV | Jsc, mA/cm2 | FF, % | PCE, % |
|---|---|---|---|---|---|
| MoOx | 5 | 1037 (1020 ± 10) | 22.3 (22.1 ± 0.2) | 76 (72 ± 4) | 17.3 (16.0 ± 1.0) |
| 10 | 1059 (1044 ± 9) | 22.2 (21.3 ± 0.8) | 77 (72 ± 2) | 18.0 (16.0 ± 1.0) | |
| 15 | 1045 (1034 ± 7) | 21.6 (20.9 ± 0.4) | 78 (74 ± 4) | 17.2 (16.0 ± 1.0) | |
| 20 | 1053 (1040 ± 50) | 22.0 (22.0 ± 1.0) | 84 (76 ± 5) | 17.5 (16.0 ± 1.0) | |
| VOx | 5 | 1014 (1007 ± 9) | 19.5 (19.4 ± 0.2) | 71 (67 ± 5) | 14.1 (13.0 ± 1.0) |
| 20 | 1028 (1020 ± 10) | 22.2 (20.3 ± 0.7) | 77 (74 ± 6) | 16.1 (16.0 ± 1.0) | |
| 30 | 1045 (1010 ± 20) | 20.7 (19.2 ± 0.8) | 78 (73 ± 4) | 15.1 (14.4 ± 0.7) | |
| 45 | 1019 (1009 ± 9) | 21.4 (20.1 ± 0.8) | 78 (67 ± 4) | 16.0 (14.0 ± 1.0) | |
| WOx | 20 | 1037 (1020 ± 10) | 22.0 (21.1 ± 0.4) | 70 (68 ± 4) | 15.7 (15.0 ± 1.0) |
| 30 | 991 (970 ± 30) | 21.3 (21.2 ± 0.1) | 68 (64 ± 3) | 14.0 (13.4 ± 0.9) | |
| 40 | 1010 (930 ± 40) | 21.5 (20.9 ± 0.8) | 70 (68 ± 4) | 15.1 (14.0 ± 2.0) | |
| 60 | 1037 (1000 ± 20) | 21.5 (20.7 ± 0.5) | 71 (63 ± 3) | 15.2 (13.0 ± 1.0) | |
| NbOx | 10 | 359 (260 ± 60) | 11.3 (10 ± 1.0) | 29 (26 ± 1) | 0.9 (0.6 ± 0.1) |
| 20 | 329 (300 ± 20) | 11.1 (10.1 ± 0.6) | 28 (26 ± 1) | 0.9 (0.8 ± 0.1) | |
| 30 | 1013 (1000 ± 300) | 21.4 (19.0 ± 2.0) | 61 (60 ± 10) | 11.9 (10.0 ± 4.0) | |
| 40 | 934 (500 ± 300) | 20.4 (16.0 ± 2.0) | 59 (40 ± 10) | 11.2 (3.0 ± 3.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tepliakova, M.M.; Mikheeva, A.N.; Somov, P.A.; Statnik, E.S.; Korsunsky, A.M.; Stevenson, K.J. Combination of Metal Oxide and Polytriarylamine: A Design Principle to Improve the Stability of Perovskite Solar Cells. Energies 2021, 14, 5115. https://doi.org/10.3390/en14165115
Tepliakova MM, Mikheeva AN, Somov PA, Statnik ES, Korsunsky AM, Stevenson KJ. Combination of Metal Oxide and Polytriarylamine: A Design Principle to Improve the Stability of Perovskite Solar Cells. Energies. 2021; 14(16):5115. https://doi.org/10.3390/en14165115
Chicago/Turabian StyleTepliakova, Marina M., Alexandra N. Mikheeva, Pavel A. Somov, Eugene S. Statnik, Alexander M. Korsunsky, and Keith J. Stevenson. 2021. "Combination of Metal Oxide and Polytriarylamine: A Design Principle to Improve the Stability of Perovskite Solar Cells" Energies 14, no. 16: 5115. https://doi.org/10.3390/en14165115
APA StyleTepliakova, M. M., Mikheeva, A. N., Somov, P. A., Statnik, E. S., Korsunsky, A. M., & Stevenson, K. J. (2021). Combination of Metal Oxide and Polytriarylamine: A Design Principle to Improve the Stability of Perovskite Solar Cells. Energies, 14(16), 5115. https://doi.org/10.3390/en14165115





.jpeg)