A Review on Advanced Manufacturing for Hydrogen Storage Applications
Abstract
:1. Introduction
2. Advanced Manufacturing Options
2.1. Advanced Manufacturing (AM) Techniques
- (a)
- Binder Jetting
- (b)
- Material Jetting
- (c)
- Material Extrusion
- (d)
- Vat Photopolymerization
- (e)
- Powder Bed Fusion
- (f)
- Direct Energy Deposition
- (g)
- Sheet Lamination
2.2. Application and Trends of 3D Printing Technologies
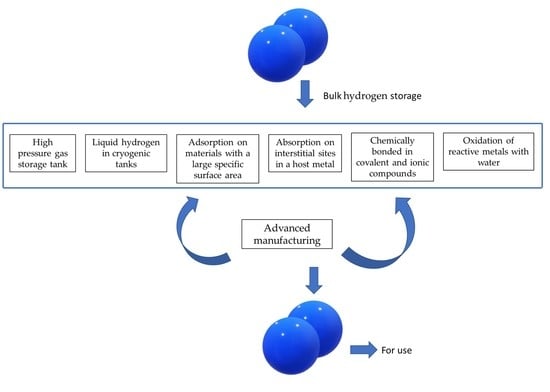
3. Hydrogen Storage Options
3.1. Mechanical Hydrogen Storage Options
3.2. Chemical Storage Options
- a.
- Metal Hydrides
- b.
- Carbon nanostructures
4. Conclusions and Recommendation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torres-Madroñero, J.L.; Alvarez-Montoya, J.; Restrepo-Montoya, D.; Tamayo-Avendaño, J.M.; Nieto-Londoño, C.; Sierra-Pérez, J. Technological and Operational Aspects That Limit Small Wind Turbines Performance. Energies 2020, 13, 6123. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, J.; Liang, H.; Zhao, Y.; Shao, Y. Hydrodynamic Responses of a 6 MW Spar-Type Floating Offshore Wind Turbine in Regular Waves and Uniform Current. Fluids 2020, 5, 187. [Google Scholar] [CrossRef]
- Sarwar, J.; Shad, M.R.; Hasnain, A.; Ali, F.; Kakosimos, K.E.; Ghosh, A. Performance Analysis and Comparison of a Concentrated Photovoltaic System with Different Phase Change Materials. Energies 2021, 14, 2911. [Google Scholar] [CrossRef]
- Ehrbar, D.; Schmocker, L.; Vetsch, D.; Boes, R. Hydropower Potential in the Periglacial Environment of Switzerland under Climate Change. Sustainability 2018, 10, 2794. [Google Scholar] [CrossRef] [Green Version]
- Araújo, K.; Shropshire, D. A Meta-Level Framework for Evaluating Resilience in Net-Zero Carbon Power Systems with Extreme Weather Events in the United States. Energies 2021, 14, 4243. [Google Scholar] [CrossRef]
- Banerjee, K.; Robb, K.R.; Radulescu, G.; Scaglione, J.M.; Wagner, J.C.; Clarity, J.B.; LeFebvre, R.A.; Peterson, J.L. Estimation of Inherent Safety Margins in Loaded Commercial Spent Nuclear Fuel Casks. Nucl. Technol. 2016, 195, 124–142. [Google Scholar] [CrossRef]
- Ding, H.; Wu, W.; Jiang, C.; Ding, Y.; Bian, W.; Hu, B.; Singh, P.; Orme, C.J.; Wang, L.; Zhang, Y.; et al. Self-Sustainable Protonic Ceramic Electrochemical Cells Using a Triple Conducting Electrode for Hydrogen and Power Production. Nat. Commun. 2020, 11, 1907. [Google Scholar] [CrossRef]
- Mondal, K.; Kumar, R.; Sharma, A. Metal-Oxide Decorated Multilayered Three-Dimensional (3D) Porous Carbon Thin Films for Supercapacitor Electrodes. Ind. Eng. Chem. Res. 2016, 55, 12569–12581. [Google Scholar] [CrossRef]
- Nagar, R.; Vinayan, B.P.; Samantaray, S.S.; Ramaprabhu, S. Recent Advances in Hydrogen Storage Using Catalytically and Chemically Modified Graphene Nanocomposites. J. Mater. Chem. A 2017, 5, 22897–22912. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Stefanakos, E.K. Clean Energy and Fuel Storage. Appl. Sci. 2019, 9, 3270. [Google Scholar] [CrossRef] [Green Version]
- Utomo, O.; Abeysekera, M.; Ugalde-Loo, C.E. Optimal Operation of a Hydrogen Storage and Fuel Cell Coupled Integrated Energy System. Sustainability 2021, 13, 3525. [Google Scholar] [CrossRef]
- Nicoletti, G. The Hydrogen Option for Energy: A Review of Technical, Environmental and Economic Aspects. Int. J. Hydrogen Energy 1995, 20, 759–765. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen Storage in Carbon Materials—A Review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Barthélémy, H. Hydrogen Storage–Industrial Prospectives. Int. J. Hydrogen Energy 2012, 37, 17364–17372. [Google Scholar] [CrossRef]
- Felderhoff, M.; Weidenthaler, C.; von Helmolt, R.; Eberle, U. Hydrogen Storage: The Remaining Scientific and Technological Challenges. Phys. Chem. Chem. Phys. 2007, 9, 2643. [Google Scholar] [CrossRef] [PubMed]
- Kreider, M.C.; Sefa, M.; Fedchak, J.A.; Scherschligt, J.; Bible, M.; Natarajan, B.; Klimov, N.N.; Miller, A.E.; Ahmed, Z.; Hartings, M.R. Toward 3D Printed Hydrogen Storage Materials Made with ABS-MOF Composites. Polym. Adv. Technol. 2018, 29, 867–873. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen Storage Methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Peng, J.-K.; Hua, T.Q. Cryo-compressed hydrogen storage. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 119–145. ISBN 978-1-78242-362-1. [Google Scholar]
- Zacharia, R.; Rather, S. Review of Solid State Hydrogen Storage Methods Adopting Different Kinds of Novel Materials. J. Nanomater. 2015, 2015, 914845. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A. Hydrogen Storage in Platinum Loaded Single-Walled Carbon Nanotubes. Int. J. Hydrogen Energy 2020, 45, 23966–23970. [Google Scholar] [CrossRef]
- Ning, F.; Cong, W.; Qiu, J.; Wei, J.; Wang, S. Additive Manufacturing of Carbon Fiber Reinforced Thermoplastic Composites Using Fused Deposition Modeling. Compos. Part B Eng. 2015, 80, 369–378. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Mondal, K.; Fujimoto, K.; McMurtrey, M.D. Advanced Manufacturing of Printed Melt Wire Chips for Cheap, Compact Passive In-Pile Temperature Sensors. JOM 2020, 72, 4196–4201. [Google Scholar] [CrossRef]
- Tripathy, P.K.; Mondal, K.; Khanolkar, A.R. One-Step Manufacturing Process for Neodymium-Iron (Magnet-Grade) Master Alloy. Mater. Sci. Energy Technol. 2021, 4, 249–255. [Google Scholar] [CrossRef]
- Mondal, K.; McMurtrey, M.D. Present Status of the Functional Advanced Micro-, Nano-Printings—A Mini Review. Mater. Today Chem. 2020, 17, 100328. [Google Scholar] [CrossRef]
- Mondal, K.; Nuñez, L.; Downey, C.M.; van Rooyen, I.J. Thermal Barrier Coatings Overview: Design, Manufacturing, and Applications in High-Temperature Industries. Ind. Eng. Chem. Res. 2021, 60, 6061–6077. [Google Scholar] [CrossRef]
- Mondal, K.; Nuñez, L.; Downey, C.M.; van Rooyen, I.J. Recent Advances in the Thermal Barrier Coatings for Extreme Environments. Mater. Sci. Energy Technol. 2021, 4, 208–210. [Google Scholar] [CrossRef]
- Srinivasan, S.; Rivera, L.; Escobar, D.; Stefanakos, E. Light Weight Complex Metal Hydrides for Reversible Hydrogen Storage. In Advanced Applications of Hydrogen and Engineering Systems in the Automotive Industry; Cocco, L., Aziz, M., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-297-1. [Google Scholar]
- Hassanpouryouzband, A.; Joonaki, E.; Edlmann, K.; Haszeldine, R. Offshore Geological Storage of Hydrogen: Is This Our Best Option to Achieve Net-Zero? ACS Energy Lett. 2021, 6, 2181–2186. [Google Scholar] [CrossRef]
- Quadrelli, E.; Centi, G.; Duplan, J.; Perathoner, S. Carbon Dioxide Recycling: Emerging Large-Scale Technologies with Industrial Potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B.; Cheremisin, A. Insights into CO2 Capture by Flue Gas Hydrate Formation: Gas Composition Evolution in Systems Containing Gas Hydrates and Gas Mixtures at Stable Pressures. ACS Sustain. Chem. Eng. 2018, 6, 5732–5736. [Google Scholar] [CrossRef]
- Sambasivarao, K.V.; Deshmukh, S.G. Selection and Implementation of Advanced Manufacturing Technologies: Classification and Literature Review of Issues. Int. J. Oper. Prod. Manag. 1995, 15, 43–62. [Google Scholar] [CrossRef]
- Chan, F.T.S.; Chan, M.H.; Lau, H.; Ip, R.W.L. Investment Appraisal Techniques for Advanced Manufacturing Technology (AMT): A Literature Review. Integr. Manuf. Syst. 2001, 12, 35–47. [Google Scholar] [CrossRef]
- Trappey, A.J.C.; Trappey, C.V.; Govindarajan, U.H.; Sun, J.J.; Chuang, A.C. A Review of Technology Standards and Patent Portfolios for Enabling Cyber-Physical Systems in Advanced Manufacturing. IEEE Access 2016, 4, 7356–7382. [Google Scholar] [CrossRef]
- Zhakeyev, A.; Wang, P.; Zhang, L.; Shu, W.; Wang, H.; Xuan, J. Additive Manufacturing: Unlocking the Evolution of Energy Materials. Adv. Sci. 2017, 4, 1700187. [Google Scholar] [CrossRef] [Green Version]
- Haleem, A.; Javaid, M. Additive Manufacturing Applications in Industry 4.0: A Review. J. Ind. Integr. Manag. 2019, 4, 1930001. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Singh, R. Material Issues in Additive Manufacturing: A Review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Bourell, D.L. Perspectives on Additive Manufacturing. Annu. Rev. Mater. Res. 2016, 46, 1–18. [Google Scholar] [CrossRef]
- Leary, M. Binder jetting. In Design for Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–339. ISBN 978-0-12-816721-2. [Google Scholar]
- Díaz-Moreno, C.A.; Lin, Y.; Hurtado-Macías, A.; Espalin, D.; Terrazas, C.A.; Murr, L.E.; Wicker, R.B. Binder Jetting Additive Manufacturing of Aluminum Nitride Components. Ceram. Int. 2019, 45, 13620–13627. [Google Scholar] [CrossRef]
- Chavez, L.A.; Ibave, P.; Wilburn, B.; Alexander, D.; Stewart, C.; Wicker, R.; Lin, Y. The Influence of Printing Parameters, Post-Processing, and Testing Conditions on the Properties of Binder Jetting Additive Manufactured Functional Ceramics. Ceramics 2020, 3, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Yap, Y.L.; Wang, C.; Sing, S.L.; Dikshit, V.; Yeong, W.Y.; Wei, J. Material Jetting Additive Manufacturing: An Experimental Study Using Designed Metrological Benchmarks. Precis. Eng. 2017, 50, 275–285. [Google Scholar] [CrossRef]
- Yang, H.; Lim, J.C.; Liu, Y.; Qi, X.; Yap, Y.L.; Dikshit, V.; Yeong, W.Y.; Wei, J. Performance Evaluation of ProJet Multi-Material Jetting 3D Printer. Virtual Phys. Prototyp. 2017, 12, 95–103. [Google Scholar] [CrossRef]
- Sireesha, M.; Lee, J.; Kranthi Kiran, A.S.; Babu, V.J.; Kee, B.B.T.; Ramakrishna, S. A Review on Additive Manufacturing and Its Way into the Oil and Gas Industry. RSC Adv. 2018, 8, 22460–22468. [Google Scholar] [CrossRef] [Green Version]
- Torrado, A.R.; Shemelya, C.M.; English, J.D.; Lin, Y.; Wicker, R.B.; Roberson, D.A. Characterizing the Effect of Additives to ABS on the Mechanical Property Anisotropy of Specimens Fabricated by Material Extrusion 3D Printing. Addit. Manuf. 2015, 6, 16–29. [Google Scholar] [CrossRef]
- Park, S.-I.; Rosen, D.W.; Choi, S.; Duty, C.E. Effective Mechanical Properties of Lattice Material Fabricated by Material Extrusion Additive Manufacturing. Addit. Manuf. 2014, 1–4, 12–23. [Google Scholar] [CrossRef]
- Hsiang Loh, G.; Pei, E.; Gonzalez-Gutierrez, J.; Monzón, M. An Overview of Material Extrusion Troubleshooting. Appl. Sci. 2020, 10, 4776. [Google Scholar] [CrossRef]
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A Review on Fabricating Tissue Scaffolds Using Vat Photopolymerization. Acta Biomater. 2018, 74, 90–111. [Google Scholar] [CrossRef]
- Monzón, M.; Ortega, Z.; Hernández, A.; Paz, R.; Ortega, F. Anisotropy of Photopolymer Parts Made by Digital Light Processing. Materials 2017, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Schmidleithner, C.; Kalaskar, D.M. Stereolithography. In 3D Printing; Cvetković, D., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-965-2. [Google Scholar]
- Vitale, A.; Cabral, J. Frontal Conversion and Uniformity in 3D Printing by Photopolymerisation. Materials 2016, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.T.; Kriewall, C.S.; Leu, M.C.; Newkirk, J.W. Powder Characterisation Techniques and Effects of Powder Characteristics on Part Properties in Powder-Bed Fusion Processes. Virtual Phys. Prototyp. 2017, 12, 3–29. [Google Scholar] [CrossRef]
- King, W.E.; Anderson, A.T.; Ferencz, R.M.; Hodge, N.E.; Kamath, C.; Khairallah, S.A.; Rubenchik, A.M. Laser Powder Bed Fusion Additive Manufacturing of Metals; Physics, Computational, and Materials Challenges. Appl. Phys. Rev. 2015, 2, 041304. [Google Scholar] [CrossRef]
- Huber, F.; Rasch, M.; Schmidt, M. Laser Powder Bed Fusion (PBF-LB/M) Process Strategies for In-Situ Alloy Formation with High-Melting Elements. Metals 2021, 11, 336. [Google Scholar] [CrossRef]
- Huber, F.; Papke, T.; Scheitler, C.; Hanrieder, L.; Merklein, M.; Schmidt, M. In Situ Formation of a Metastable β-Ti Alloy by Laser Powder Bed Fusion (L-PBF) of Vanadium and Iron Modified Ti-6Al-4V. Metals 2018, 8, 1067. [Google Scholar] [CrossRef] [Green Version]
- Javidani, M.; Arreguin-Zavala, J.; Danovitch, J.; Tian, Y.; Brochu, M. Additive Manufacturing of AlSi10Mg Alloy Using Direct Energy Deposition: Microstructure and Hardness Characterization. J. Therm. Spray Technol. 2017, 26, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Shim, D.-S.; Baek, G.-Y.; Seo, J.-S.; Shin, G.-Y.; Kim, K.-P.; Lee, K.-Y. Effect of Layer Thickness Setting on Deposition Characteristics in Direct Energy Deposition (DED) Process. Opt. Laser Technol. 2016, 86, 69–78. [Google Scholar] [CrossRef]
- Baek, G.Y.; Shin, G.Y.; Lee, K.Y.; Shim, D.S. Mechanical Properties of Tool Steels with High Wear Resistance via Directed Energy Deposition. Metals 2019, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Tolochko, N.K.; Sokol, O.V. Assessing the Economic Effectiveness of Additive Sheet Lamination. Russ. Eng. Res. 2021, 41, 5–9. [Google Scholar] [CrossRef]
- Bhatt, P.M.; Kabir, A.M.; Peralta, M.; Bruck, H.A.; Gupta, S.K. A Robotic Cell for Performing Sheet Lamination-Based Additive Manufacturing. Addit. Manuf. 2019, 27, 278–289. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Sheet Lamination Processes. In Additive Manufacturing Technologies; Springer: Boston, MA, USA, 2010; pp. 223–252. ISBN 978-1-4419-1119-3. [Google Scholar]
- Chin, S.Y.; Dikshit, V.; Meera Priyadarshini, B.; Zhang, Y. Powder-Based 3D Printing for the Fabrication of Device with Micro and Mesoscale Features. Micromachines 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, R.; Cieslik, J.; Kulpa, M.; Dudek, P.; Zagorski, K.; Rumin, R. Comparison of Cost, Material and Time Usage in FDM and SLS 3D Printing Methods. In Proceedings of the 2017 XIIIth International Conference on Perspective Technologies and Methods in MEMS Design (MEMSTECH), Lviv, Ukraine, 20–23 April 2017; pp. 12–14. [Google Scholar]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.-F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Campbell, T.; Williams, C.; Ivanova, O.; Garrett, B. Could 3D Printing Change the World? Atlantic Council: Washington, DC, USA, 2011. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Hao, B.; Lin, G. 3D Printing Technology and Its Application in Industrial Manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 782, 022065. [Google Scholar] [CrossRef]
- Raghavendra Prabhu, S.; Ilangkumaran, M.; Mohanraj, T. 3D Printing of Automobile Spoilers Using MCDM Techniques. Mater. Test. 2020, 62, 1121–1125. [Google Scholar] [CrossRef]
- Joshi, S.C.; Sheikh, A.A. 3D Printing in Aerospace and Its Long-Term Sustainability. Virtual Phys. Prototyp. 2015, 10, 175–185. [Google Scholar] [CrossRef]
- Deters, J. 3D-printing impacts on systems engineering in defense industry. In Additive Manufacturing Handbook; CRC Press: Boca Raton, FL, USA, 2017; pp. 49–56. ISBN 1-315-11910-2. [Google Scholar]
- Liaw, C.-Y.; Guvendiren, M. Current and Emerging Applications of 3D Printing in Medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Zeng, L.; Li, P.; Yao, Y.; Niu, B.; Niu, S.; Xu, B. Recent Progresses of 3D Printing Technologies for Structural Energy Storage Devices. Mater. Today Nano 2020, 12, 100094. [Google Scholar] [CrossRef]
- Mathur, R. 3D Printing in Architecture. Int. J. Innov. Sci. Eng. Technol. 2016, 3, 583–591. [Google Scholar]
- Holt, C.; Edwards, L.; Keyte, L.; Moghaddam, F.; Townsend, B. Construction 3D Printing. In 3D Concrete Printing Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 349–370. ISBN 978-0-12-815481-6. [Google Scholar]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.P.; Kim, J.; Han, M.S. The Convergence of Three-Dimensional Printing and Nail-Art Technology. J. Cosmet. Med. 2019, 3, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Vanderploeg, A.; Lee, S.-E.; Mamp, M. The Application of 3D Printing Technology in the Fashion Industry. Int. J. Fash. Des. Technol. Educ. 2017, 10, 170–179. [Google Scholar] [CrossRef]
- Yu, J. The Application of 3D Printing Technology in Sculpture. In Proceedings of the 2020 International Conference on Machine Learning and Big Data Analytics for IoT Security and Privacy, Shanghai, China, 6–8 November 2020; MacIntyre, J., Zhao, J., Ma, X., Eds.; Advances in Intelligent Systems and Computing. Springer International Publishing: Cham, Switzerland, 2021; Volume 1283, pp. 755–759, ISBN 978-3-030-62745-4. [Google Scholar]
- Leinonen, T.; Virnes, M.; Hietala, I.; Brinck, J. 3D Printing in the Wild: Adopting Digital Fabrication in Elementary School Education. Int. J. Art Des. Educ. 2020, 39, 600–615. [Google Scholar] [CrossRef]
- Varsha Shree, M.; Dhinakaran, V.; Rajkumar, V.; Bupathi Ram, P.M.; Vijayakumar, M.D.; Sathish, T. Effect of 3D Printing on Supply Chain Management. Mater. Today Proc. 2020, 21, 958–963. [Google Scholar] [CrossRef]
- Manero, A.; Smith, P.; Sparkman, J.; Dombrowski, M.; Courbin, D.; Kester, A.; Womack, I.; Chi, A. Implementation of 3D Printing Technology in the Field of Prosthetics: Past, Present, and Future. Int. J. Environ. Res. Public Health 2019, 16, 1641. [Google Scholar] [CrossRef] [Green Version]
- Oladapo, B.I.; Ismail, S.O.; Afolalu, T.D.; Olawade, D.B.; Zahedi, M. Review on 3D Printing: Fight against COVID-19. Mater. Chem. Phys. 2021, 258, 123943. [Google Scholar] [CrossRef] [PubMed]
- Oropallo, W.; Piegl, L.A. Ten Challenges in 3D Printing. Eng. Comput. 2016, 32, 135–148. [Google Scholar] [CrossRef]
- Martins, J.P.; Ferreira, M.P.A.; Ezazi, N.Z.; Hirvonen, J.T.; Santos, H.A.; Thrivikraman, G.; França, C.M.; Athirasala, A.; Tahayeri, A.; Bertassoni, L.E. 3D printing: Prospects and challenges. In Nanotechnologies in Preventive and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 299–379. ISBN 978-0-323-48063-5. [Google Scholar]
- Tetsuka, H.; Shin, S.R. Materials and Technical Innovations in 3D Printing in Biomedical Applications. J. Mater. Chem. B 2020, 8, 2930–2950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D Printing Technologies for Electrochemical Energy Storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Cheng, M.; Deivanayagam, R.; Shahbazian-Yassar, R. 3D Printing of Electrochemical Energy Storage Devices: A Review of Printing Techniques and Electrode/Electrolyte Architectures. Batter. Supercaps 2020, 3, 130–146. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Heier, J.; Zhang, C.J. Printing and Coating MXenes for Electrochemical Energy Storage Devices. J. Phys. Energy 2020, 2, 031004. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Li, B.; Chen, Z.; Mi, S.; Lao, C. Fabrication and Characterization of 3D-Printed Highly-Porous 3D LiFePO4 Electrodes by Low Temperature Direct Writing Process. Materials 2017, 10, 934. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Wei, C.; Yu, L.; Xia, Z.; Cai, J.; Tian, Z.; Zou, G.; Dou, S.X.; Sun, J. 3D Printing of Porous Nitrogen-Doped Ti3C2 MXene Scaffolds for High-Performance Sodium-Ion Hybrid Capacitors. ACS Nano 2020, 14, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen Production Technologies-Membrane Based Separation, Storage and Challenges. J. Environ. Manag. 2022, 302, 113963. [Google Scholar] [CrossRef]
- Lawson, S.; Alwakwak, A.-A.; Rownaghi, A.A.; Rezaei, F. Gel–Print–Grow: A New Way of 3D Printing Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 56108–56117. [Google Scholar] [CrossRef]
- Hou, J.; Sapnik, A.F.; Bennett, T.D. Metal–Organic Framework Gels and Monoliths. Chem. Sci. 2020, 11, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Chakraborty, A.; Mondal, K.; Chandrasekhar, V. Multi-Ruthenocene Assemblies on an Organostannoxane Platform. Supramolecular Signatures and Conversion to (Ru–Sn)O2. Cryst. Growth Des. 2014, 14, 861–870. [Google Scholar] [CrossRef]
- Lim, G.J.H.; Wu, Y.; Shah, B.B.; Koh, J.J.; Liu, C.K.; Zhao, D.; Cheetham, A.K.; Wang, J.; Ding, J. 3D-Printing of Pure Metal–Organic Framework Monoliths. ACS Mater. Lett. 2019, 1, 147–153. [Google Scholar] [CrossRef]
- Philamore, H.; Rossiter, J.; Walters, P.; Winfield, J.; Ieropoulos, I. Cast and 3D Printed Ion Exchange Membranes for Monolithic Microbial Fuel Cell Fabrication. J. Power Sources 2015, 289, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Luo, H.; Li, R.; Wang, L.; Man, C.; Li, X. Superior Resistance to Hydrogen Damage for Selective Laser Melted 316L Stainless Steel in a Proton Exchange Membrane Fuel Cell Environment. Corros. Sci. 2020, 166, 108425. [Google Scholar] [CrossRef]
- Li, Z.; Wang, A.; Mahtab, M.; Chen, K.; Wang, J. Application of the 3D-Printing and Commercial Off-The-Shelf Components in the Design of a Micro-Propulsion System. In Proceedings of the 31st Annual AIAA/USU Conference on Small Satellites, Logan, UT, USA, 5–10 August 2017. [Google Scholar]
- Hirscher, M.; Yartys, V.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.; Broom, D.; Buckley, C.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Makridis Hydrogen storage and compression. In Methane and Hydrogen for Energy Storage; Carriveau, T. (Ed.) Institution of Engineering and Technology: London, UK, 2016; pp. 1–28. ISBN 978-1-78561-193-3. [Google Scholar]
- Nash, D.; Aklil, D.; Johnson, E.; Gazey, R.; Ortisi, V. Hydrogen Storage. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 131–155. ISBN 978-0-08-087873-7. [Google Scholar]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef]
- Züttel, A. Materials for Hydrogen Storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Norton, J.R.; Sowa, J. Introduction: Metal Hydrides. Chem. Rev. 2016, 116, 8315–8317. [Google Scholar] [CrossRef] [Green Version]
- Graetz, J. Metastable Metal Hydrides for Hydrogen Storage. ISRN Mater. Sci. 2012, 2012, 863025. [Google Scholar] [CrossRef] [Green Version]
- Wormald, J.; Zerkle, M.; Holmes, J. Generation of the TSL for Zirconium Hydrides from Ab Initio Methods. J. Nucl. Eng. 2021, 2, 105–113. [Google Scholar] [CrossRef]
- Kim, K.C.; Kulkarni, A.D.; Johnson, J.K.; Sholl, D.S. Large-Scale Screening of Metal Hydrides for Hydrogen Storage from First-Principles Calculations Based on Equilibrium Reaction Thermodynamics. Phys. Chem. Chem. Phys. 2011, 13, 7218. [Google Scholar] [CrossRef] [PubMed]
- Mu, S. Electrospun Nanofibrous Materials and Their Hydrogen Storage. In Hydrogen Storage; Liu, J., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0731-6. [Google Scholar]
- Mondal, K.; Maitra, T.; Srivastava, A.K.; Pawar, G.; McMurtrey, M.D.; Sharma, A. 110th Anniversary: Particle Size Effect on Enhanced Graphitization and Electrical Conductivity of Suspended Gold/Carbon Composite Nanofibers. Ind. Eng. Chem. Res. 2020, 59, 1944–1952. [Google Scholar] [CrossRef]
- Mondal, K.; Balasubramaniam, B.; Gupta, A.; Lahcen, A.A.; Kwiatkowski, M. Carbon Nanostructures for Energy and Sensing Applications. J. Nanotechnol. 2019, 2019, 1454327. [Google Scholar] [CrossRef] [Green Version]
- Mondal, K.; Pawar, G.; McMurtrey, M.D.; Sharma, A. Finetuning Hierarchical Energy Material Microstructure via High Temperature Material Synthesis Route. Mater. Today Chem. 2020, 16, 100269. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, A.; Singh, C.; Sumana, G.; Malhotra, B.D.; Sharma, A. Highly Sensitive Porous Carbon and Metal/Carbon Conducting Nanofiber Based Enzymatic Biosensors for Triglyceride Detection. Sens. Actuators B Chem. 2017, 246, 202–214. [Google Scholar] [CrossRef]
- Katiyar, S.; Mondal, K.; Sharma, A. One-Step Sol–Gel Synthesis of Hierarchically Porous, Flow-through Carbon/Silica Monoliths. RSC Adv. 2016, 6, 12298–12310. [Google Scholar] [CrossRef]
- Kishnani, V.; Yadav, A.; Mondal, K.; Gupta, A. Palladium-Functionalized Graphene for Hydrogen Sensing Performance: Theoretical Studies. Energies 2021, 14, 5738. [Google Scholar] [CrossRef]
- Mondal, K.; Gupta, A. Recent Advances in Carbon–Semiconductor Nanocomposites for Water Remediation. In Water Remediation; Bhattacharya, S., Gupta, A.B., Gupta, A., Pandey, A., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 45–74. ISBN 978-981-10-7550-6. [Google Scholar]
- Mondal, K. Recent Advances in the Synthesis of Metal Oxide Nanofibers and Their Environmental Remediation Applications. Inventions 2017, 2, 9. [Google Scholar] [CrossRef]
- Mondal, K.; Kumar, J.; Sharma, A. Self-Organized Macroporous Thin Carbon Films for Supported Metal Catalysis. Colloids Surf. Physicochem. Eng. Asp. 2013, 427, 83–94. [Google Scholar] [CrossRef]
- Assfour, B.; Leoni, S.; Seifert, G.; Baburin, I.A. Packings of Carbon Nanotubes-New Materials for Hydrogen Storage. Adv. Mater. 2011, 23, 1237–1241. [Google Scholar] [CrossRef]
- Yang, S. Understanding Covalent Grafting of Nanotubes onto Polymer Nanocomposites: Molecular Dynamics Simulation Study. Sensors 2021, 21, 2621. [Google Scholar] [CrossRef]
- Xing, Y.; Fang, B.; Bonakdarpour, A.; Zhang, S.; Wilkinson, D. Facile fabrication of mesoporous carbon nanofibers with unique hierarchical nanoarchitecture for electrochemical hydrogen storage. Int. J. Hydrogen Energy 2014, 39, 7859–7867. [Google Scholar] [CrossRef]
- Mondal, K.; Tripathy, P. Preparation of Smart Materials by Additive Manufacturing Technologies: A Review. Materials 2021, 14, 6442. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.; Suman, R. Significant roles of 4D printing using smart materials in the field of manufacturing. Adv. Ind. Eng. Polym. Res. 2021, 4, 301–311. [Google Scholar] [CrossRef]













| AM Technique | Material | Advantage | Disadvantage |
|---|---|---|---|
| Fused Deposition Modeling (FDM) | Thermoplastics such as carbon nanofiber/AB S, PLA, PA Nylon, Graphene/AB S | Low cost, high speed, deposition of various materials, printed parts that are multi-functional. | Thermoplastic polymer as the only working material, material should be in filament form, absence of homogeneity in disperse material. |
| Stereolithography (SLA) | Photocurable TiO2/epoxy acrylate, CNT/acrylic ester BST/epoxy | Best for making of concept prototypes, quick manufacturing times and decent surface appearance and geometrical accuracy | Restricted to procedure non-functional materials, for example resins or plastics, Materials are costly and inadequate in accessibility, unable to process functional materials such as metals, and needs support structures. |
| Binder Jetting (BJ) | Aluminum oxide (Al2O3) and alumina-silica powders, PCL, PLA polymers and binder materials, amorphous or colloidal silicon carbide (SiC) | Decent printing resolution, soft materials with multi color ability, and low cost processing compared to SLM, SLA printing techniques | Mechanical strength of printed parts is not decent, high surface roughness, existence of large porous microstructures in manufactured parts. |
| Sheet Lamination (SL) | Any sheet material foil such as paper, metals, plastics, fibers glass, composite | Appropriate for processing of medium and large sized constituents, such as dies or metal making tools and extensive choice of obtainable materials in the form of sheet form, no obligation of pre-designed support structure, it is a faster technique. | Poor layer bonding carries the risk of de-lamination, Strength of the produced components in the perpendicular path to the layers is considerablly less than in additional directions and several post processing methods are required, post processing is a must, manufacturing time increases as no. of layers grows |
| Direct Energy Deposition (DED) | HSS, Tool steel, nickel—based alloys titanium aluminum | Layer can be made-up in any alignment, range of materials in the form of powder can be handled, large parts can be fabricated and improved deposition rates are conceivable | Geometrical accuracy is inferior, stair-stepping consequence can constrain geometric precision and post-processing might be needed |
| Selective laser sintering (SLS) | PCL and polyimide powder, carbon black/nylon-12, Al2O3/polystyrene, silica/nylon | Materials which can be managed include plastics, ceramics, sands and some metals, parts shaped are appropriate for functional testing and no support structures are needed | Accessibility of metallic materials is limited; a surrounded chamber is essential and metal sintering brings porosity and mechanically weak components produced. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Free, Z.; Hernandez, M.; Mashal, M.; Mondal, K. A Review on Advanced Manufacturing for Hydrogen Storage Applications. Energies 2021, 14, 8513. https://doi.org/10.3390/en14248513
Free Z, Hernandez M, Mashal M, Mondal K. A Review on Advanced Manufacturing for Hydrogen Storage Applications. Energies. 2021; 14(24):8513. https://doi.org/10.3390/en14248513
Chicago/Turabian StyleFree, Zach, Maya Hernandez, Mustafa Mashal, and Kunal Mondal. 2021. "A Review on Advanced Manufacturing for Hydrogen Storage Applications" Energies 14, no. 24: 8513. https://doi.org/10.3390/en14248513
APA StyleFree, Z., Hernandez, M., Mashal, M., & Mondal, K. (2021). A Review on Advanced Manufacturing for Hydrogen Storage Applications. Energies, 14(24), 8513. https://doi.org/10.3390/en14248513