1. Introduction
Polymer flooding is an important technology to extend oilfield life and increase oilfield production [
1,
2,
3,
4,
5,
6,
7,
8]. As the most commonly used polymer in field applications, a small quantity of partially hydrolyzed polyacrylamide (HPAM) can increase the viscosity of water by 2 or more orders of magnitude in the absence of added electrolytes because of the extremely high molecular weight and the repulsion between the negative charges along the polymer chain [
9]. Employing polymer flooding for enhancement of oil recovery has major advantages, but also considerable drawbacks [
10]. One such drawback is the lack of stability in terms of viscous and viscoelastic properties of HPAM caused by shearing actions of the stirrer, pipeline valve, injection pump, shot hole, and porous media during preparation and injection of polymer solution. One of the most common and obvious effects of this instability is significantly reduced viscosity of HPAM solutions when its linear architecture undergoes such scission, thus seriously reducing its ability to enhance oil recovery [
8,
10,
11,
12,
13,
14,
15,
16].
Several different polymeric topologies have been explored for their chain scission resistance characteristics, and such efforts largely appear to have been directed by availability of the architecture. For example, branching or grafting side chains to polymeric backbones is considered to enhance shear stability. However, grafting onto polymeric backbones may enhance the tensile stresses due to the additional drag on the grafts, and thus reduce shear stability [
14].
With multiple linear chains linked to a single core, hyperbranched polymers have an elementary branching topology, which could enhance shear resistance. This is attributed to sacrificial scission of the branches, leading to only a small decrease in molecular weight. Currently, organics are usually used as cores to synthesize hyperbranched polymers [
17,
18,
19,
20], but they cannot effectively increase the number of branched chains on the premise of meeting the requirement for injection. Inorganic nano-SiO
2 is of small grain size, and has an internal three-dimensional, reticular, rigid structure, with many highly active Si–OH radical groups on its surface [
21]. Prior researchers have introduced nano-SiO
2 into oil-displacing polymers by means of surface modification [
9,
22,
23,
24,
25]. Pu et al. [
23] synthesized novel water-soluble core-shell hyperbranched polymers (HBPAMs), consisting of nano-SiO
2 as the core, hyperbranched polyamidoamine (PAMAM) as the subshell, and linear hydrophilic chains as the outermost layer. Static experiments convincingly proved that the three-dimensional morphology endowed HBPAMs with excellent shear degradation resistance. The author’s research group has synthesized a hybrid hyperbranched polymer based on nano-SiO
2, modified by 3-(trimethoxysilyl)-1-propanamine and maleic anhydride, successively [
25].
By varying the numbers of functionalized branch-cell units, the numbers of outmost linear hydrophilic chains can be tuned, which will affect the copolymer’s shear resistance [
23]. Consequently, the primary objective of this study was to: (1) synthesize two copolymers with different degrees of modification by using the previously synthesized polymerizable modified nano-SiO
2 monomers [
25] as the core; and (2) determine the shear resistance of the copolymers and the impact of the modification degree on the shear resistance. With the purpose of simulating the shearing actions the occur during preparation and injection in practice, the shearing resistance of the copolymer solutions were evaluated by mechanical shearing and porous media shearing, respectively. In this study, we: (1) analyzed the shear resistance of the copolymers from the perspective of their molecular structure based upon their hydrodynamic radius, weight-average molecular weight, and microstructure; and (2) researched the macro-rheological property and dynamic viscoelasticity of the copolymer before/after shearing to assess the shear stability of the copolymer solution [
10]. Finally, indoor mobility control experiments were carried out on the copolymer that was found to have better shear stability in terms of viscosity and rheological property to further investigate its feasibility for enhance oil recovery (EOR).
2. Materials and Methods
2.1. Reagents and Materials
Modified nano-SiO
2 was prepared by the method provided in the references [
25]. Acrylamide (AM), acrylic acid (AA), absolute ethyl alcohol (C
2H
5OH), ammonium persulfate ((NH
4)
2S
2O
8), sodium hydrogen sulfite (NaHSO
3), sodium hydroxide (NaOH), NaCl, MgCl
2·6H
2O, CaCl
2, and other chemicals were purchased from Chengdu KeLong Chemical Reagent Co., Ltd. (Chengdu, China), were of analytical grade, and used without further purification. Partially hydrolyzed polyacrylamide (HPAM, 10% degree of hydrolysis, M
w = 1.2 × 10
7) was obtained from Sichuan Guangya Polymer Chemical Co., Ltd. (Chengdu, China).
2.2. Synthesis of Modified Nano-SiO2/AA/AM Copolymer
A certain amount of modified nano-SiO
2 (degree of modification: 26% and 38% corresponding to HPMNS-1 and HPMNS-2, respectively), AA, and AM were placed in a 100 mL three-necked flask. An appropriate amount of distilled water was added into the flask to prepare an aqueous solution with monomers having a mass concentration of 26%. After that, the pH value of the solution was adjusted to 7.4 with sodium hydroxide. Then, ammonium persulfate solution and sodium hydrogen sulfite solution (molar ratio: 1:1) were added into the flask. Copolymerization was performed at 40 °C under nitrogen atmosphere for an indicated time. Finally, modified nano-SiO
2/AA/AM copolymer (HPMNS) was obtained by washing (with absolute ethyl alcohol), smashing, and drying the reaction products. The synthesis route of the copolymer is shown in
Scheme 1.
2.3. Characterization
Infrared (IR) spectra of the HPMNS were measured with KBr pellets using a Perkin Elmer RX-1 spectrophotometer (Beijing Rayleigh Analytical Instrument, Beijing, China) in the optical range of 4400–500 cm−1. 1H-NMR and 13C-NMR were recorded on Bruker AC-E 200 spectrometer (Bruker BioSpin, Faellanden, Switzerland) at 400 MHz with D2O and CCl4 solvent, respectively. The hydrodynamic diameter (Rh) and distribution of the samples were obtained using a BI-200SM wide-angle dynamic laser light scattering (DLS) instrument. Static laser scattering (SLS) measured the weight-average molecular weight (Mw) and radius of gyration (Rg) of the polymers with the concentration of 30–70 mg/L. The morphologies of the samples before/after shearing were observed by Quanta 450 Environment Scanning Electron Microscope (ESEM, FEI Company, Hillsboro, OR, USA).
2.4. Shear Resistance Test
Polymer (HPAM, HPMNS-1, and HPMNS-2) was dissolved to 2000 mg/L by the brine, whose components are listed in
Supplementary Materials, Table S1. The viscosity of HPMNS was determined at different shearing rates on HAAKE RS 600 rotational rheometer at 70 °C. Moreover, the polymer solutions were sheared at different shear strengths by a Mixing Speed Governor (WT-VSA2000B) and different flow rates by a porous media shearing model (inner diameter: 0.01 m; length: 0.235 m; packing: 80–100 mesh quartz sand), which were employed to simulate the mechanical shear degradation of the pipeline and wellbore, and the shear of porous media, respectively. Then the viscosity of the sheared and unsheared polymer solutions was detected by Brookfield DV-III viscometer at 70 °C.
2.5. Rheological Property
The macro-rheological properties of polymer solutions (2000 mg/L by the brine) before/after being sheared by the Mixing Speed Governor (3600 r/min, 20 s) and porous media shearing model (injection speed: 40.4 mL/min) were measured by a HAAKE RS 600 rotational rheometer at 70 °C. The shearing rate was 0.01–400.00 s−1 and the measurement duration was 6 min. Simultaneously, HAAKE RS 600 was employed to determine the elastic modulus (G’) and viscous modulus (G’’) of the samples in order to obtain their viscoelasticity properties.
2.6. Mobility Control Capacity
The sand-packed tube displacement experiments were carried out as described in reference [
26]. The tubes (30 cm in length and 2.5 cm in inner diameter) were packed with 100–120 mesh quartz sand that was washed with an 18% hydrochloric acid solution and then with a massive amount of water until the pH reached 7. The brine with a TDS (total dissolved salt) of 9784 mg·L
−1 was used in the experiments whose ionic composition is shown in
Supplementary Materials, Table S1. The flooding rate for water flooding and polymer flooding was set as 1.0 mL/min and kept unchanged. The mobility control ability of polymer was characterized by resistance factor (RF) and residual resistance factor (RRF). The RF and the RRF were calculated by the following equations [
22,
27]:
where
Kw and
Kp are the aqueous and polymer phase permeability, respectively (×10
−3 μm
2),
μw and
μp are the aqueous and polymer viscosity, respectively (mPa·s), and
Kwb and
Kwa are the aqueous phase permeability before and after polymer flooding, respectively, where
Kwb =
KW (×10
−3 μm
2).
3. Results and Discussion
3.1. IR Spectra, 1H-NMR Spectra, and 13H-NMR Spectra Analysis
The structure of HPMNS was confirmed by IR spectra, as shown in
Figure 1a. In the spectrum curve of the copolymer, the peak at 1115 cm
−1 and 785.2 cm
−1 were attributed to the antisymmetric stretching vibration and symmetrical stretching vibration of Si–O–Si, respectively. The peak at 3419.7 cm
−1 was attributed to the stretching vibration peak of N–H. The peak at 2940.6 cm
−1 was assigned to the stretching vibration of C–H in the methylene. The peaks at 1672.2 cm
−1 were the stretching vibration peaks of C=O. The results show that the target copolymer was successfully synthesized.
Figure 1b shows the
1H NMR spectrum of HPMNS. The chemical shift value at 1.57 ppm was assigned to the methylene (–C
H2–) protons in the polymeric main chain (–C
H2–CH(COONa)–, –C
H2–CH(CONH
2)–, –C
H(COONa)–CH(CONH–)–), and the parts of the modified nano-SiO
2 (–C
H2–C
H2–CH
2(CONH)–), respectively. The chemical shift value at 2.11 ppm was due to the methyne (–C
H–) protons in the polymeric main chain (–CH
2–C
H(COONa)–, –CH
2–C
H(CONH
2)–, and –CH(COONa)–C
H(CONH–)–). The peak at 2.49 ppm identified the methylene (–C
H2–) protons on the part attaching to the acylamino of the modified nano-SiO
2 (–CH
2–CH
2–C
H2(CONH)–).
The
13C-NMR spectrum of HPMNS is shown in
Figure 1c. The peaks at 29.94 ppm, 34.38 ppm, and 39.83 ppm were assigned to the carbon in the modified nano-SiO
2 (–
CH
2–
CH
2–
CH
2(CONH)–), successively. The chemical shift value at 35.13 ppm, 45.27 ppm, and 182.90 ppm were the part of acrylic acid in the polymeric main chain (–
CH
2–
CH(
COONa)–), successively. The peak at 35.69 ppm, 41.96 ppm, and 179.64 ppm were the part of acrylamide in the polymeric main chain (–
CH
2–
CH(
CONH
2)–), successively. The peak at 36.99 ppm was the part of modified nano-SiO
2 in the polymeric main chain (–
CH(COONa)–
CH(CONH–)–). The chemical shift value at 179.64 ppm was due to the carbon of the acylamino in the modified nano-SiO
2 (–CH(COONa)–CH(
CONH–)–). Finally, the peak at 183.83 ppm was attributable to carbon of the carboxyl in the modified nano-SiO
2 (–CH(
COONa)–CH(CONH–)–). Hence, the structure of HPMNS was in accordance with the anticipated polymer molecular structure based on the
1H-NMR and
13C-NMR data.
3.2. Weight-Average Molecular Weight and Flexibility of HPMNS
The weight-average molecular weight and radius of gyration of polymer solutions were determined through a static light scattering test.
Figure S1a–c (Supplementary Materials) show Zimm plots of HPAM, HPMNS-1, and HPMNS-2, respectively. On the basis of fundamentals of molecular weight determination by static light scattering, the weight-average molecular weight and radius of gyration of polymers are shown in
Table 1.
The flexibility of the polymers’ molecular chain was characterized by the unperturbed dimension of the polymers. The unperturbed dimension of the polymers was obtained based upon their radius of gyration and molecular weight according to Equations (1) and (2) [
28], as shown in
Table 1.
where
A is unperturbed dimension of polymer (nm(mol/g)
1/2),
is mean square end-to-end distance, (nm
2), and M is molecular weight of polymer (g/mol).
The unperturbed dimension of polymer represents the unperturbed mean square end-to-end distance of unit molecular weight which cannot be directly measured, but the mean square radius of gyration
, which is used to characterize the molecular dimension, can be directly measured by experiment. Mathematical justification showed that when the molecular weight is infinite, the relationship between the mean square end-to-end distance and mean square radius of gyration of the freely jointed chain and equivalent freely jointed chain are as follows:
where
is mean square radius of gyration (nm
2).
In
Table 1, among the three polymers, HPMNS-2 had the highest flexibility, followed by HPMNS-1 and HPAM. This result is due to the molecular chains of HPAM comprising a C–C single bond. In the modified nano-SiO
2/AA/AM copolymer, the 3-aminopropyl trimethoxysilane molecular structure used for nano-SiO
2 modification contains a C–Si single bond and C–N single bond, which have fewer barriers to internal rotation compared with the C–C single bond, thus resulting in easier conformational transitions and more conformations [
29]. Similarly, because of a higher degree of modification, the proportion of C–Si single bonds and C–N single bonds in the molecular chains of HPMNS-2 was higher than that of HPMNS-1.
3.3. Hydrodynamic Radius of HPMNS
The hydrodynamic radius of HPAM (281 nm), HPMNS-1 (198 nm), and HPMNS-2 (163 nm) are shown in
Figure S2a–c (Supplementary Materials), respectively. Compared to HPAM, which was of linear structure and existed in the solution in the form of random molecular coil, HPMNS, which was of hyperbranched structure with a network structure in the solution, had more contracted conformations. Hence, HPMNS had a shorter hydrodynamic radius than HPAM. In addition, HPMNS-2 has a higher degree of modification, more branched chains, lower molecular weight, and finer structure than HPMNS-1, thus its hydrodynamic radius was the shortest.
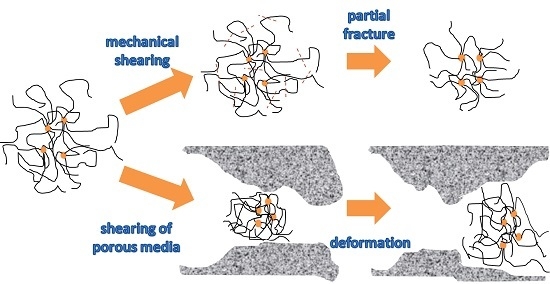
3.4. Shear Resistance of HPMNS
During the injection process, the polymer viscosity will decrease owing to shear degradation occurred in injection pipelines, equipment, perforation nozzles, and near well bore [
12]. Hence, we needed to carry out experimental evaluations for the shear resistance of polymers.
In this study, the shear recovery curve of the copolymer is shown in
Figure 2. The viscosity retention ratio of HPMNS solution was 84.9% after it was sheared at the speed of 500 s
−1 for 5 min. This indicated that the copolymer had some shear resistance.
The viscosity retention ratios of polymer solutions sheared by the Mixing Speed Governor at diverse shear strengths are shown in
Table 2. Compared with HPAM solution, HPMNS possessed higher viscosity retention ratios under the same conditions, and as the shearing strength increased, the decreasing trend of the viscosity retention ratio of HPMNS decreased. Thus, HPMNS showed some shear resistance. Moreover, HPMNS-2 with a higher degree of modification, had better shear resistance.
As shown in
Table 3, the shearing by porous media had great impacts on the viscosity of polymer solutions. The higher the injection velocity was, the lower the viscosity retention ratio of polymer solutions was. The viscosity retention ratio of HPAM was 53.25% at an injection velocity of 40.4 mL/min. When the velocity was increased to 80.8 mL/min, the viscosity retention ratio was as low as 28.46%. Nevertheless, compared with HPAM solution, HPMNS enjoyed higher viscosity retention ratios under the same conditions. Besides, HPMNS-2 showed better shear resistance with a viscosity retention ratio of 60.12%, while that of HPMNS-1 and HPAM was 58.48% and 53.25%, respectively, at a fixed injection velocity of 40.4 mL/min.
According to the above results, HPMNS-2 showed the best shear resistance, followed by HPMNS-1 and HPAM, to mechanical shear action and porous media shear action. From the perspective of molecular structure, owing to their having the highest flexibility, the molecular chains of HPMNS-2 could deform according to the pore-throat size when the polymer solutions flowed through porous media, thus suffering from less damage than HPMNS-1 and HPAM at the same degree of blockage. Similarly, the molecular chain conformations of HPMNS-2 solution were contracted due to this molecule’s short hydrodynamic radius. The proportion of its damaged molecular chains decreased, and the impact of shear action on hydrodynamic radius was reduced. In addition, when the hydrodynamic radius was small, it was easier for the solutions to flow through the pore throat of porous media. Therefore, the viscosity retention ratios of HPAM, HPMNS-1, and HPMNS-2 solutions rose successively after they were sheared by the Mixing Speed Governor and porous media.
3.5. Morphology of HPMNS
To investigate microstructures of HPAM, HPMNS-1, and HPMNS-2, ESEM was utilized on unsheared and sheared (by the Mixing Speed Governor) polymer solutions. As can be seen from
Figure 3a, before shearing, vast molecular coils were entangled with each other, thus forming some network structures in HPAM solution. After shearing, the coil size reduced and the network structures in the solution became weaker (
Figure 3b). On the contrary, finer network structures were formed in HPMNS-1 and HPMNS-2 sheared solutions (as shown in
Figure 3d,f), which was beneficial for enhancing the acting force among molecular chains and protecting molecular chains from being more damaged. This phenomena is due to the hyperbranched structures of HPMNS in solution; the coil size of the molecule was reduced, but the molecular coils were still entangled after shearing. Therefore, HPMNS had higher viscosity retention ratios than HPAM under the same shear strength.
The network structures in HPMNS-2 solution were more compact than those in HPMNS-1 due to a higher degree of modification. HPMNS-2 had more branched chains, which were beneficial for enhancing the acting force among molecular chains and consequently intensifying the entanglement of molecular chains. In addition, HPMNS-2 had a shorter hydrodynamic radius, more contracted conformations, and a lower proportion of damaged molecular chains at the same shear strength, which could form more compact network structures after shearing. This accounts for the better shearing resistance of HPMNS-2 compared with HPMNS-1.
3.6. Rheological Property of HPMNS
The rheological behavior of a polymer solution is very important for its application in enhanced oil recovery [
9]. With a good viscoelastic performance, the polymer can effectively displace small oil blocks in the dead angles of formation [
30]. Hence, from the perspective of rheological behavior, we assessed the shear stability of HPMNS by studying the macro-rheological property and dynamic viscoelasticity of sheared and unsheared polymer solutions.
The variation of the apparent viscosity was curved based on the shearing rate, as shown in
Figure 4. HPMNS and HPAM solutions before (
Figure 4a) and after shearing (
Figure 4b,c) all showed shear-thinning behaviors, which is beneficial from the standpoint of injectivity. Because the viscosity near the injection well is lower due to higher shear rate, there is a more favorable injectivity. Once the polymer moves far into the reservoir, shear rates decline and the viscosity increases, which provides the desired mobility control [
31].
At low shearing rates, the viscosities of the sheared and unsheared HPMNS solutions were all higher than those of HPAM. This was due to HPMNS being a hyperbranched polymer that can form stronger structures in the solution. In addition, with good shear resistance, the impact of shear action on HPMNS solution viscosity was less than HPAM, giving it better stability in terms of rheological behavior after being sheared by the Mixing Speed Governor and porous media shearing model.
Compared with HPMNS-2, the unsheared HPMNS-1 solution had higher viscosities at low shearing rates. The cause might be that the conformational changes of molecular chains in the polymers were very slow when the shearing rates were low, as they needed to overcome great internal frictional resistance. On the other hand, HPMNS-1 and HPMNS-2 had similar structures before shearing. Under this circumstance, the larger the hydrodynamic radius was, the higher the internal frictional resistance was. Thus, having the higher molecular weight and larger hydrodynamic radius, HPMNS-1 solution had higher viscosity. This phenomenon was opposite in the sheared solution, where HPMNS-2 had higher viscosity than HPMNS-1 at low shearing rates, as shown in
Figure 4b,c. This could be attributed to HPMNS-2 having a better shear resistance.
The dynamic viscoelasticity of the polymer solutions is shown in
Figure 5 (scanning stress: 0.10 Pa, scanning frequency: 0.1–15.0 Hz). At the scanning frequency of 0.1–4.6 Hz, the viscosity modulus (G’’) of the sheared and unsheared polymer solutions were all higher than its elasticity modulus (G’), which was dominated by viscosity. From
Figure 5a, the values of G’ and G’’ of unsheared HPMNS-1 solution were both higher than those of HPMNS-2, followed by those of HPAM. With better shearing resistance, HPMNS-2 could be comparable to HPMNS-1 in terms of viscosity modulus and elasticity modulus, after being sheared by the Mixing Speed Governor (
Figure 5b). When passing through the pore throat of porous media, HPMNS-2 was more flexible than HPMNS-1, since it could deform and then recover to some extent. By this way, the values of G’ and G’’ for HPMNS-2 increased (
Figure 5c).
3.7. Mobility Control Capacity of HPMNS
The basic idea behind using water-soluble polymers in many oilfield operations and various enhanced oil recovery processes is to reduce the mobility of the aqueous phase and consequently to improve the sweep efficiency [
32,
33]. Due to the good thickening ability of the sheared HPMNS-2 solution, reduction of the water–oil mobility ratio can be achieved by increasing the viscosity of the water phase [
27]. Furthermore, on the basis of mobility control theory, increasing the residual resistance factor not only reduces the water–oil mobility ratio, but also decreases the requirement for viscosity enhancement of the polymer solution [
34]. In view of this, we studied the residual resistance factor caused by HPMNS-2, which had better shear stability than HPMNS-1, to investigate its feasibility for EOR.
With a permeability of K = 300 × 10
−3 μm
2, the injection pressure curves of HPMNS-2 and HPAM solution in different concentrations (1000, 2000 mg/L) are shown in
Figure 6. In an oil reservoir with a permeability of K = 300 × 10
−3 μm
2, the injection pressures of the copolymer, with concentrations of 1000 mg/L and 2000 mg/L, were less than 0.6 MPa and 1.8 MPa, respectively. The polymer solution was injected after the differential pressure of water flooding became stable and the water-phase permeability was determined. The differential pressure increased rapidly at the beginning and then slowed down. After the differential pressure became stable again, we started water flooding. At this time, the differential pressure fell rapidly and finally became stable. This means that the copolymer solutions had good injectivity in a low-to-medium permeability oil reservoir. As the injection increased, the pressure at the inlet of the homogeneous model rose gently, and reached the peak when the injection volume was about 15 PV. Thereafter, the injection pressure fell to a steady level. It can be seen from the study that the polymer solutions had good mobility control capacities in the porous media with a low-to-medium permeability.
The RF and RFF of polymer solutions were calculated according to the data of the injection pressure curves, as shown in
Table 4. The RF and the RFF of HPMNS-2 solutions (1000 mg/L and 2000 mg/L) were both higher than those of HPAM under the same conditions. This means that polymer solutions could establish higher RF and RFF and had a stronger mobility control capacity that was favorable to enhanced oil recovery.
4. Conclusions
The modified nano-SiO2/AA/AM copolymer was synthesized by free-radical polymerization. Fourier-transformation infrared (FTIR), 1H-NMR and 13C-NMR, DLS, SLS, and ESEM measurements confirmed its chemical structure and morphology. The experiments with different shearing models convincingly proved that compared with HPAM, which has been widely used in EOR, the hyperbranched structure provided the modified nano-SiO2/AA/AM copolymers with excellent shear resistance. From a microstructural point, the observation results of ESEM showed that the damage to molecular chains of the copolymer caused by shearing was less. Moreover, the higher the degree of modification was, the better shear resistance there was. The rheological properties of HPMNS solutions showed shear-thinning behavior, and sheared HPMNS-2 solutions had the highest viscosity at a low shearing rate. At a scanning stress of 0.10 Pa, the viscoelasticity of both HPMNS copolymers before/after shearing were higher than that of HPAM. Furthermore, compared to HPAM, the copolymers could establish higher RF and RFF in a medium-porous medium under a similar permeability, and had stronger mobility control capacity that could effectively improve the EOR.