Boronic Acid as Glucose-Sensitive Agent Regulates Drug Delivery for Diabetes Treatment
Abstract
:1. Introduction
2. PBA-Functionalized Materials and Polymers
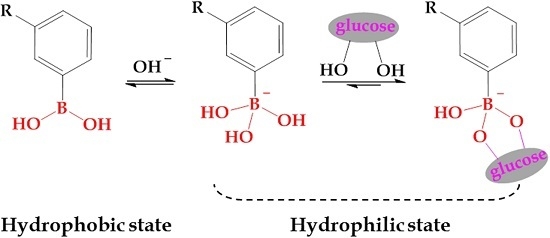
3. Glucose-Sensitivity
4. Glucose-Triggered Drug Delivery
5. Conclusions
- (i)
- Simple preparation of PBA-based glucose-sensitive drug platforms. These drug platforms must be prepared efficiently and economically in a simple way with repeated and controlled structures for different preparations. The facile and repeatable preparation of glucose-sensitive drug carriers could maintain the properties of the structure and glucose-sensitivity of drug delivery platforms. More importantly, the glucose-triggered controlled drug release and hypoglycemic effects could also be stabilized. These are very useful to promote the application of a glucose-sensitive drug delivery system in clinical diabetic therapy.
- (ii)
- Excellent glucose-sensitivity under physiological pH in diabetic blood glucose levels. Even though there are many methods to decrease the pKa value of PBA derivatives to promote the glucose-sensitivity of the carriers under physiological pH, the glucose-induced drug delivery in diabetic blood glucose levels remains a challenge, which restricts clinical application for diabetes treatment.
- (iii)
- Proof of reproducibility of glucose-sensitivity over multiple recycles. The glucose-triggered drug releases depend on the glucose levels and the loading capacity of drug. At the same drug-loading capacity, a high blood glucose level could induce a greater release of insulin, and the released insulin could lower the concentration of glucose in blood. However, the relationship between the further glucose-triggered insulin release and the hypoglycemic effects of released insulin has been studied rarely, which is very important for the long-term treatment of diabetes. How to accurately control the same amount of drug under decreasing drug concentration gradients restricts the application of glucose-sensitive drug delivery.
- (iv)
- Guarantee of the bioactivity of released drug (e.g., insulin). The payload used to reduce the blood glucose level may be degenerated and inactivated during the preparation of the drug-loaded carriers and the drug release process, which has no hypoglycemic effects. The payload must maintain the original bioactivity to guarantee hypoglycemic effects.
- (v)
- Biocompatibility and biodegradability of the carriers. Medical diabetes treatment is a long process needing safe matrices for in vivo application. The matrices must be non-toxic and friendly to the body. Great biocompatible and biodegradable materials, such as polypeptides, should be chosen in the glucose-sensitive self-regulated drug delivery systems. Polypeptides used in the human body could be degraded into small molecules of amino acids which are nontoxic toward humans.
Acknowledgments
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes—Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hasebe, Y.; Takahashi, S.; Sato, K.; Anzai, J.I. Layer-by-layer deposited nano- and micro-assemblies for insulin delivery: A review. Mater. Sci. Eng. C 2014, 34, 384–392. [Google Scholar] [CrossRef]
- Siegel, R.A. Stimuli sensitive polymers and self regulated drug delivery systems: A very partial review. J. Control. Release 2014, 190, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, F.; Sun, Y.; Gao, M.; Chai, Z. Development of shell cross-linked nanoparticles based on boronic acid-related reactions for self-regulated insulin delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, H. Parameter study of glucose-sensitive hydrogel: Effect of immobilized glucose oxidase on diffusion and deformation. Soft Matter 2013, 11, 69–74. [Google Scholar] [CrossRef]
- Sahota, T.; Sawicka, K.; Taylor, J.; Tanna, S. Effect of varying molecular weight of dextran on acrylic-derivatized dextran and concanavalin a glucose-responsive materials for closed-loop insulin delivery. Drug Dev. Ind. Pharm. 2011, 37, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, R.; Liu, G.; Liu, X.; Gao, Y.; Shen, J.; An, Y.; Shi, L. Effect of coordination on the glucose-responsiveness of PEG-b-(PAA-co-PAAPBA) micelles. Macromol. Rapid Commun. 2010, 31, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wu, Z.; Zhang, X.; Sun, L.; Li, C. Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological ph. Soft Matter 2014, 10, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic acid building blocks: Tools for self assembly. Chem. Commun. 2011, 47, 1124–1150. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ikeda, S.; Harada, A.; Kataoka, K. Glucose-responsive polymer bearing a novel phenylborate derivative as a glucose-sensing moiety operating at physiological pH conditions. Biomacromolecules 2003, 4, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiao, C.; Wang, L.; Gai, G.; Ding, J. Glucose-sensitive polymer nanoparticles for self-regulated drug delivery. Chem. Commun. 2016, 52, 7633–7652. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Nam, J.H.; Kim, Y.J.; Cho, Y.J.; Kwon, N.H.; Lee, J.Y.; Kang, H.J.; Kim, H.T.; Park, H.M.; Kim, S.; et al. Synthesis of peo-based glucose-sensitive block copolymers and their application for preparation of superparamagnetic iron oxide nanoparticles. Macromol. Res. 2011, 19, 827–834. [Google Scholar] [CrossRef]
- Cambre, J.N.; Sumerlin, B.S. Biomedical applications of boronic acid polymers. Polymer 2011, 52, 4631–4643. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, H.; He, Q.; Li, Y.; Gu, J.; Li, L.; Li, H.; Shi, J. A glucose-responsive controlled release of insulin system based on enzyme multilayers-coated mesoporous silica particles. Chem. Commun. 2011, 47, 9459–9461. [Google Scholar] [CrossRef] [PubMed]
- Ravaine, V.; Ancla, C.; Catargi, B. Chemically controlled closed-loop insulin delivery. J. Control. Release 2008, 132, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Bremner, D.H.; Li, H.Y.; Sun, X.Z.; Zhu, L.M. Synthesis and evaluation of temperature- and glucose-sensitive nanoparticles based on phenylboronic acid and N-vinylcaprolactam for insulin delivery. Mater. Sci. Eng. C 2016, 69, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Ran, M.; Huang, H.; Zhang, L.; Li, X.; Chen, M.; Akashi, M. Preparation of glucose responsive polyelectrolyte capsules with shell crosslinking via the layer-by-layer technique and sustained release of insulin. Polym. Chem. 2016, 7, 6779–6788. [Google Scholar] [CrossRef]
- Siegel, R.A.; Gu, Y.; Lei, M.; Baldi, A.; Nuxoll, E.E.; Ziaie, B. Hard and soft micro- and nanofabrication: An integrated approach to hydrogel-based biosensing and drug delivery. J. Control. Release 2010, 141, 303–313. [Google Scholar] [CrossRef]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Peppas, N.A. Is there a future in glucose-sensitive, responsive insulin delivery systems? J. Drug Deliv. Sci. Technol. 2004, 14, 247–256. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y. Boronic acid-containing hydrogels: Synthesis and their applications. Chem. Soc. Rev. 2013, 42, 8106–8121. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, Z.H.; Wu, W.; Zhou, S.; Siddiq, M. Engineering of phenylboronic acid based glucose-sensitive microgels with 4-vinylpyridine for working at physiological ph and temperature. Macromol. Chem. Phys. 2011, 212, 1510–1514. [Google Scholar] [CrossRef]
- Xu, J.; Yang, D.; Li, W.; Gao, Y.; Chen, H.; Li, H. Phenylboronate-diol crosslinked polymer gels with reversible sol-gel transition. Polymer 2011, 52, 4268–4276. [Google Scholar] [CrossRef]
- Mandal, D.; Mandal, S.K.; Ghosh, M.; Das, P.K. Phenylboronic acid appended pyrene-based low-molecular-weight injectable hydrogel: Glucose-stimulated insulin release. Chem. Eur. J. 2015, 21, 12042–12052. [Google Scholar] [CrossRef] [PubMed]
- Ancla, C.; Lapeyre, V.; Gosse, I.; Catargi, B.; Ravaine, V. Designed glucose-responsive microgels with selective shrinking behavior. Langmuir 2011, 27, 12693–12701. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Guan, Y.; Zhang, Y. Kinetics of glucose-induced swelling of P(NIPAM-AAPBA) microgels. Macromolecules 2011, 44, 4479–4486. [Google Scholar] [CrossRef]
- Lapeyre, V.; Renaudie, N.; Dechezelles, J.F.; Saadaoui, H.; Ravaine, S.; Ravaine, V. Multiresponsive hybrid microgels and hollow capsules with a layered structure. Langmuir 2009, 25, 4659–4667. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Guan, Y.; Zhang, Y. Contraction-type glucose-sensitive microgel functionalized with a 2-substituted phenylboronic acid ligand. Polym. Chem. 2014, 5, 1782–1790. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, L.; Gu, Y.-Y.; Chu, L.-Y. Microfluidic fabrication of monodisperse microcapsules for glucose-response at physiological temperature. Soft Matter 2013, 9, 4150–4159. [Google Scholar] [CrossRef]
- Lapeyre, V.; Gosse, I.; Chevreux, S.; Ravaine, V. Monodispersed glucose-responsive microgels operating at physiological salinity. Biomacromolecules 2006, 7, 3356–3363. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Luo, Q.; Guan, Y.; Zhang, Y. Drug release kinetics from monolayer films of glucose-sensitive microgel. Polymer 2010, 51, 2668–2675. [Google Scholar] [CrossRef]
- Sato, K.; Yoshida, K.; Takahashi, S.; Anzai, J. pH- and sugar-sensitive layer-by-layer films and microcapsules for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 809–821. [Google Scholar] [CrossRef]
- De Geest, B.G.; Jonas, A.M.; Demeester, J.; De Smedt, S.C. Glucose-responsive polyelectrolyte capsules. Langmuir 2006, 22, 5070–5074. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Shi, L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Polym. Chem. 2014, 5, 1503–1518. [Google Scholar] [CrossRef]
- Wang, B.; Ma, R.; Liu, G.; Li, Y.; Liu, X.; An, Y.; Shi, L. Glucose-responsive micelles from self-assembly of poly(ethylene glycol)-b-poly(acrylic acid-co-acrylamidophenylboronic acid) and the controlled release of insulin. Langmuir 2009, 25, 12522–12528. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xing, Z.; Yan, J.; Li, L.; Zhao, H.; Zha, L. Glucose and temperature dual stimuli responsiveness of intelligent hollow nanogels. Chin. J. Mater. Res. 2012, 26, 44–48. [Google Scholar]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.S.; Durfee, P.N.; Theron, C.; Ashley, C.E.; Carnes, E.C.; Brinker, C.J. Protocells: Modular mesoporous silica nanoparticle-supported lipid bilayers for drug delivery. Small 2016, 12, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Chakraborty, A.; Jana, N.R. Dextran-gated, multifunctional mesoporous nanoparticle for glucose-responsive and targeted drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 22183–22191. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; He, D.; Cai, L.; He, X.; Wang, K.; Yang, X.; Li, L.; Li, S.; Su, X. Alizarin complexone functionalized mesoporous silica nanoparticles: A smart system integrating glucose-responsive double-drugs release and real-time monitoring capabilities. ACS Appl. Mater. Interfaces 2016, 8, 8358–8366. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Zheng, C.; Wu, Z.; Li, C. A ph gated, glucose-sensitive nanoparticle based on worm-like mesoporous silica for controlled insulin release. J. Phys. Chem. B 2013, 117, 3852–3860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic amp. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Pept. Sci. 2010, 94, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-C.; Zhao, S.; Yang, B.-Y.; Wang, Q.-H.; Kuang, H.-X. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr. Polym. 2016, 148, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Yeul, V.S.; Rayalu, S.S. Unprecedented chitin and chitosan: A chemical overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, S.; Zhang, X.; Shu, S.; Chu, T.; Yu, D. Phenylboronic acid grafted chitosan as a glucose-sensitive vehicle for controlled insulin release. J. Pharm. Sci. 2011, 100, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guo, Q.; Chu, T.; Zhang, X.; Wu, Z.; Yu, D. Glucose-sensitive polyelectrolyte nanocapsules based on layer-by-layer technique for protein drug delivery. J. Mater. Sci. Mater. Med. 2013, 25, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Wu, Z.; Zheng, C.; Li, C. Oral glucose- and ph-sensitive nanocarriers for simulating insulin release in vivo. Polym. Chem. 2014, 5, 1999–2009. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, Z.; Ma, L.; Shi, C.; Shen, T.; Song, J. Fabrication of boronic acid-functionalized nanoparticles via boronic acid-diol complexation for drug delivery. RSC Adv. 2014, 4, 53877–53884. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, Z.; Wang, N.; Sun, Y.; Yan, Y.; Gao, L. Multiple sensitivity study of boronic acid-functionalized nanoparticles based on the complexation of poly(3-methacrylamido phenylboronic acid) and dextran. J. Macromol. Sci. Part A 2015, 52, 267–272. [Google Scholar] [CrossRef]
- Wang, Y.; Chai, Z.; Wang, N.; Ren, X.; Gao, M. Preparation and responsive behaviors of chitosan-functionalized nanoparticles via a boronic acid-related reaction. J. Biomater. Sci. Polym. Ed. 2015, 26, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Guo, Q.; Wu, Z.; Sun, L.; Zhang, Z.; Li, C.; Zhang, X. Amphiphilic glycopolymer nanoparticles as vehicles for nasal delivery of peptides and proteins. Eur. J. Pharm. Sci. 2013, 49, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, X.; Wang, Y.; Sun, L.; Li, C. Phenylboronic acid-containing block copolymers: Synthesis, self-assembly, and application for intracellular delivery of proteins. New J. Chem. 2012, 36, 1413–1421. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zheng, C.; Li, C. Phenylboronic acid-functionalized glycopolymeric nanoparticles for biomacromolecules delivery across nasal respiratory. Eur. J. Pharm. Biopharm. 2012, 82, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, X.; Wu, Z.; Teng, D.; Zhang, X.; Wang, Y.; Wang, Z.; Li, C. Amphiphilic random glycopolymer based on phenylboronic acid: Synthesis, characterization, and potential as glucose-sensitive matrix. Biomacromolecules 2009, 10, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yao, Q.; Cai, C.; Gou, J.; Zhang, Y.; Zhong, H.; Tang, X. Amphiphilic poly(amino acid) based micelles applied to drug delivery: The in vitro and in vivo challenges and the corresponding potential strategies. J. Control. Release 2015, 199, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Lalatsa, A.; Schätzlein, A.G.; Mazza, M.; Le, T.B.H.; Uchegbu, I.F. Amphiphilic poly(L-amino acids)—New materials for drug delivery. J. Control. Release 2012, 161, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jianxun, D.; Chunsheng, X.; Xuesi, C.; Guangqing, G.; Liyan, W. Poly(l-glutamic acid) microsphere: Preparation and application in oral drug controlled release. Acta Chim. Sin. 2015, 73, 60–65. [Google Scholar]
- Zhang, Y.; Xiao, C.; Ding, J.; Li, M.; Chen, X.; Tang, Z.; Zhuang, X.; Chen, X. A comparative study of linear, y-shaped and linear-dendritic methoxy poly(ethylene glycol)-block-polyamidoamine-block-poly(l-glutamic acid) block copolymers for doxorubicin delivery in vitro and in vivo. Acta Biomater. 2016, 40, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, C.; Zhang, Y.; Xu, W.; Wang, J.; Chen, X. Chirality-mediated polypeptide micelles for regulated drug delivery. Acta Biomater. 2015, 11, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, A.A.; Park, K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials 1997, 18, 801–806. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, J.; Xiao, C.; He, P.; Tang, Z.; Pang, X.; Zhuang, X.; Chen, X. Glucose-sensitive polypeptide micelles for self-regulated insulin release at physiological ph. J. Mater. Chem. 2012, 22, 12319–12328. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, C.; Ding, J.; Zhuang, X.; Gai, G.; Wang, L.; Chen, X. Competitive binding-accelerated insulin release from a polypeptide nanogel for potential therapy of diabetes. Polym. Chem. 2015, 6, 3807–3815. [Google Scholar] [CrossRef]
- Liu, G.; Ma, R.; Ren, J.; Li, Z.; Zhang, H.; Zhang, Z.; An, Y.; Shi, L. A glucose-responsive complex polymeric micelle enabling repeated on–off release and insulin protection. Soft Matter 2013, 9, 1636–1644. [Google Scholar] [CrossRef]
- Yang, H.; Ma, R.; Yue, J.; Li, C.; Liu, Y.; An, Y.; Shi, L. A facile strategy to fabricate glucose-responsive vesicles via a template of thermo-sensitive micelles. Polym. Chem. 2015, 6, 3837–3846. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Li, C.; Liu, Y.; An, Y.; Ma, R.; Shi, L. Glucose-responsive polymer vesicles templated by α-CD/PEG inclusion complex. Biomacromolecules 2015, 16, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choe, K.; Jeong, Y.; Yoo, J.; Lee, S.M.; Park, J.H.; Kim, P.; Kim, Y.C. Establishment of a controlled insulin delivery system using a glucose-responsive double-layered nanogel. RSC Adv. 2015, 5, 14482–14491. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Y.; Zhang, Y. Dynamically bonded layer-by-layer films for self-regulated insulin release. J. Mater. Chem. 2012, 22, 16299–16305. [Google Scholar] [CrossRef]
- Ding, Z.; Guan, Y.; Zhang, Y.; Zhu, X.X. Layer-by-layer multilayer films linked with reversible boronate ester bonds with glucose-sensitivity under physiological conditions. Soft Matter 2009, 5, 2302–2309. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, Y.; Zhou, S. Synthesis and volume phase transitions of glucose-sensitive microgels. Biomacromolecules 2006, 7, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Yamamoto, K.; Yoshida, R.; Kataoka, K.; Aoyagi, T.; Miyahara, Y. A totally synthetic glucose responsive gel operating in physiological aqueous conditions. Chem. Commun. 2010, 46, 2203–2205. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ishii, T.; Nishida, J.; Matsumoto, H.; Kataoka, K.; Miyahara, Y. A synthetic approach toward a self-regulated insulin delivery system. Angew. Chem. Int. Ed. 2012, 51, 2124–2128. [Google Scholar] [CrossRef] [PubMed]
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic acid building blocks: Tools for sensing and separation. Chem. Commun. 2011, 47, 1106–1123. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, M.; Wang, L.; Gao, C.; Xi, S. A novel triple-responsive poly(3-acrylamidephenylboronic acid-co-2-(dimethylamino) ethyl methacrylate)/(β-cyclodextrin-epichlorohydrin)hydrogels: Synthesis and controlled drug delivery. React. Funct. Polym. 2011, 71, 666–673. [Google Scholar] [CrossRef]
- Hoare, T.; Pelton, R. Charge-switching, amphoteric glucose-responsive microgels with physiological swelling activity. Biomacromolecules 2008, 9, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Mitra, N.; Yan, E.C.Y.; Zhou, S. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. Acs Nano 2010, 4, 4831–4839. [Google Scholar] [CrossRef] [PubMed]
- Gordijo, C.R.; Shuhendler, A.J.; Wu, X.Y. Glucose-responsive bioinorganic nanohybrid membrane for self-regulated insulin release. Adv. Funct. Mater. 2010, 20, 1404–1412. [Google Scholar] [CrossRef]
- Ma, R.; Yang, H.; Li, Z.; Liu, G.; Sun, X.; Liu, X.; An, Y.; Shi, L. Phenylboronic acid-based complex micelles with enhanced glucose-responsiveness at physiological pH by complexation with glycopolymer. Biomacromolecules 2012, 13, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, G.; Du, X.; Chen, H.; Liu, Y.; Huang, Q.; Kong, X.; Yao, J. Preparation of glucose-responsive and fluorescent micelles via a combination of raft polymerization and chemoenzymatic transesterification for controlled release of insulin. RSC Adv. 2015, 5, 75766–75772. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, T.; Chen, H.; Li, L.; Liu, Y.; Zhou, H.; Feng, Y.; Zhou, J. Preparation of multi-responsive micelles for controlled release of insulin. Colloid Polym. Sci. 2015, 293, 209–215. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, C.; Ding, J.; He, P.; Tang, Z.; Pang, X.; Zhuang, X.; Chen, X. Facile one-pot synthesis of glucose-sensitive nanogel via thiol-ene click chemistry for self-regulated drug delivery. Acta Biomater. 2013, 9, 6535–6543. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Guo, H.; Li, C.; Yu, D. An injectable and glucose-sensitive nanogel for controlled insulin release. J. Mater. Chem. 2012, 22, 22788–22796. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Huang, Q.; Liu, Y.; Wang, Q.; Wang, L.; Xiao, S.; Bi, F.; Ding, J. Boronic Acid as Glucose-Sensitive Agent Regulates Drug Delivery for Diabetes Treatment. Materials 2017, 10, 170. https://doi.org/10.3390/ma10020170
Zhao L, Huang Q, Liu Y, Wang Q, Wang L, Xiao S, Bi F, Ding J. Boronic Acid as Glucose-Sensitive Agent Regulates Drug Delivery for Diabetes Treatment. Materials. 2017; 10(2):170. https://doi.org/10.3390/ma10020170
Chicago/Turabian StyleZhao, Li, Qiongwei Huang, Yangyang Liu, Qing Wang, Liyan Wang, Shanshan Xiao, Fei Bi, and Jianxun Ding. 2017. "Boronic Acid as Glucose-Sensitive Agent Regulates Drug Delivery for Diabetes Treatment" Materials 10, no. 2: 170. https://doi.org/10.3390/ma10020170
APA StyleZhao, L., Huang, Q., Liu, Y., Wang, Q., Wang, L., Xiao, S., Bi, F., & Ding, J. (2017). Boronic Acid as Glucose-Sensitive Agent Regulates Drug Delivery for Diabetes Treatment. Materials, 10(2), 170. https://doi.org/10.3390/ma10020170