Photoactive Nanomaterials Inspired by Nature: LTL Zeolite Doped with Laser Dyes as Artificial Light Harvesting Systems
Abstract
:1. Introduction
2. Results and Discussion
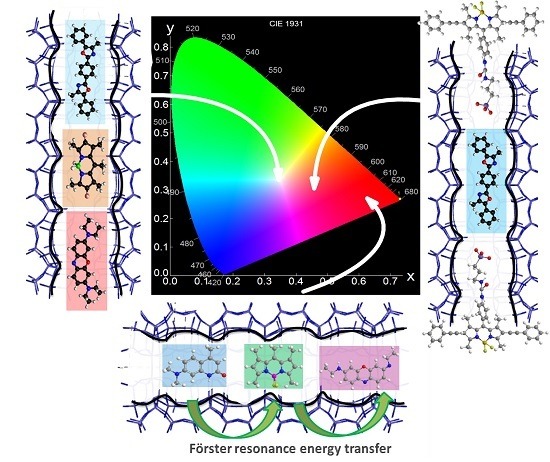
2.1. Energy Transfer across the Channels of Dye-Doped LTL Zeolite
2.1.1. Dmpopop, PM567,Ox1-Doped LTL Zeolite
2.1.2. C165,PM546,Ox4-Doped LTL Zeolite
2.2. External Functionalization of LTL Zeolite
3. Materials and Methods
3.1. Dyes
3.2. LTL Zeolite Synthesis
3.3. Dye Incorporation
3.4. External Functionalization of LTL Zeolite
3.5. Dye Loading Level
3.6. Spectroscopic and Microscopic Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Benniston, A.C.; Harriman, A. Artificial photosynthesis. Mater. Today 2008, 11, 26–34. [Google Scholar] [CrossRef]
- El-Khouly, M.; El-Mohsnawy, E.; Fukuzumi, S. Solar energy conversion: From natural to artificial photosynthesis. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 36–83. [Google Scholar] [CrossRef]
- Zan, G.; Wu, Q. Biomimetic and bioinspired synthesis of nanomaterials/nanostructures. Adv. Mater. 2016, 28, 2099–2147. [Google Scholar] [CrossRef]
- Scholes, G.D.; Mirkovic, T.; Turner, D.B.; Fassioli, F.; Buchleitner, A. Solar light harvesting by energy transfer: From ecology to coherence. Energy Environ. Sci. 2012, 5, 9374–9393. [Google Scholar] [CrossRef]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Hambourger, M.; Moore, G.F.; Kramer, D.M.; Gust, D.; Moore, A.L.; Moore, T.A. Biology and technology for photochemical fuel production. Chem. Soc. Rev. 2009, 38, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Del Guerzo, A.; Olive, A.G.L.; Reichwagen, J.; Hopf, H.; Desvergne, J.P. Energy transfer in self-assembled [n]-acene fibers involving ≥100 donors per acceptor. J. Am. Chem. Soc. 2005, 127, 17984–17985. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Aratani, N.; Osuka, A. Cyclic porphyrin arrays as artificial photosynthetic antenna: Synthesis and excitation energy transfer. Chem. Soc. Rev. 2007, 36, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Puntoriero, F.; Sartorel, A.; Orlandi, M.; La Ganga, G.; Serroni, S.; Bonchio, M.; Scandola, F.; Campagna, S. Photoinduced water oxidation using dendrimeric Ru(II) complexes as photosensitizers. Coord. Chem. Rev. 2011, 255, 2594–2601. [Google Scholar] [CrossRef]
- Dutta, P.K.; Varghese, R.; Nangreave, J.; Lin, S.; Yan, H.; Liu, Y. DNA-directed artificial light-harvesting antenna. J. Am. Chem. Soc. 2011, 133, 11985–11993. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.N.; Jang, P.; Meyer, T.J.; Papanikolas, J.M. Energy migration dynamics in a Ru(II)- and Os(II)-based antenna polymer embedded in a disordered, rigid medium. J. Phys. Chem. B 2004, 108, 2205–2209. [Google Scholar] [CrossRef]
- Martínez-Martínez, V.; García, R.; Gómez-Hortigüela, L.; Sola Llano, R.; Pérez-Pariente, J.; López-Arbeloa, I. Highly luminescent and optically switchable hybrid material by one-pot encapsulation of dyes into MgAPO-11 unidirectional nanopores. ACS Photonics 2014, 1, 205–211. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Singapore, 2006. [Google Scholar]
- Hötzer, B.; Medintz, I.L.; Hildebrandt, N. Fluorescence in nanobiotechnology: Sophisticated fluorophores for novel applications. Small 2012, 8, 2297–2326. [Google Scholar] [CrossRef] [PubMed]
- Claassens, N.J.; Volpers, M.; Santos, V.; van der Oost, J.; de Vos, W.M. Potential of proton-pumping rhodopsins: Engineering photosystems into microorganisms. Trends Biotechnol. 2013, 31, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ding, X.; Chen, L.; Wu, Y.; Liu, L.; Addicoat, M.; Irle, S.; Dong, Y.; Jiang, D. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci. Rep. 2016, 6, 32944. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V. Controlling photochemical reactions via confinement: Zeolites. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 145–466. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Abrams, L.; Kaneko, K. Mesopore-modified zeolites: Preparations, characterization and applications. Chem. Rev. 2006, 106, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Brackmann, U. Laser Dyes, 3rd ed.; Lambda Physik AG: Göttingen, Germany, 2000. [Google Scholar]
- Schulz-Ekloff, G.; Wöhrle, D.; Van Duffel, B.; Schoonheydt, R.A. Chromphores in porous silicas and minerals: Preparation and optical properties. Microporous Mesoporous Mater. 2002, 51, 91–138. [Google Scholar] [CrossRef]
- Calzaferri, G. Nanochannels: Hosts for the supramolecular organization of molecules and complexes. Langmuir 2012, 28, 6216–6231. [Google Scholar] [CrossRef] [PubMed]
- Sola-Llano, R.; Martínez-Martínez, V.; Fujita, Y.; Gómez-Hortigüela, L.; Alfayate, A.; Uji-i, H.; Fron, E.; Pérez-Pariente, J.; López-Arbeloa, I. Formation of a nonlinear optical host–guest hybrid material by tight confinement of LDS 722 into aluminophosphate 1D nanochannels. Chem. Eur. J. 2016, 22, 15700–15711. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Díaz, U.; García, T.; Sastre, G.; Velty, A. Multifunctional hybrid organic−inorganic catalytic materials with a hierarchical system of well-defined micro- and mesopores. J. Am.Chem. Soc. 2010, 132, 15011–15021. [Google Scholar] [CrossRef] [PubMed]
- Larlus, O.; Valtchev, V.P. Crystal morphology control of LTL-type zeolite crystals. Chem. Mater. 2004, 16, 3381–3389. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave synthesis of nanoporous materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Itani, L.; Bozhilov, K.N.; Clet, G.; Delmotte, L.; Valtchev, V. Factors that control zeolite L crystal size. Chem. Eur. J. 2011, 17, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Lupulescu, A.I.; Kumar, M.; Rimer, J.D. A facile strategy to design zeolite L crystals with tunable morphology and surface architecture. J. Am. Chem. Soc. 2013, 135, 6608–6617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Li, R.; Rimer, J.D. Assembly and evolution of amorphous precursors in zeolite L crystallization. Chem. Mater. 2016, 28, 1714–1727. [Google Scholar] [CrossRef]
- Devaux, A.; Calzaferri, G.; Bleser, P.; Cao, P.; Brüwhiler, D.; Kunsmann, A. Efficient and robust host-guest antenna composite for light harvesting. Chem. Mater. 2014, 26, 6878–6885. [Google Scholar] [CrossRef]
- Tabachi, G.; Calzaferri, G.; Fois, E. One-dimensional self-assembly of perylene-diimide dyes by unidirectional transit of zeolite channel openings. Chem. Commun. 2016, 52, 11195–11198. [Google Scholar] [CrossRef] [PubMed]
- Insuwan, W.; Rangsriwatananon, K.; Meeprasert, J.; Namuangruk, S.; Surakhot, Y.; Kungwan, N.; Jungsuttiwong, S. Combined experimental and theoretical investigation on fluorescence resonance energu transfer of dye loaded on LTL zeolite. Microporous Mesoporous Mater. 2017, 241, 372–382. [Google Scholar] [CrossRef]
- Gartzia-Rivero, L.; Bañuelos-Prieto, J.; Martínez-Martínez, V.; López-Arbeloa, I. Versatile photoactive materials based on zeolite L doped with laser dyes. ChemPlusChem 2012, 77, 61–70. [Google Scholar] [CrossRef]
- Vohra, V.; Calzaferri, G.; Destri, S.; Pasini, M.; Porzio, W.; Botta, C. Towards white light emission through efficient two-step energy transfer in hybrid nanofibers. ACS Nano 2010, 4, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Giasante, C.; Schäfer, C.; Raffy, G.; Del Guerzo, A. Exploiting direct and cascade energy transfer for color-tunable and white-light emission in three-component self-assembled nanofibers. J. Phys. Chem. C 2012, 116, 21706–21716. [Google Scholar] [CrossRef]
- Sens, R.; Drexhäge, K.H. Fluorescence quantum yield of oxazine and carbazine laser dyes. J. Lumin. 1981, 24/25, 709–712. [Google Scholar] [CrossRef]
- Gartzia-Rivero, L.; Cerdán, L.; Bañuelos, J.; Enciso, E.; López-Arbeloa, I.; Costela, A.; García-Moreno, I. Förster resonance energy transfer and laser efficiency in colloidal suspensions of dye-doped nanoparticles: Concentration effects. J. Phys. Chem. C 2014, 118, 13107–13117. [Google Scholar] [CrossRef]
- Brühwiler, D.; Calzaferri, G. Selective functionalizaion of the external surface of zeolite L. C. R. Chim. 2005, 8, 391–398. [Google Scholar] [CrossRef]
- Tabachi, G.; Fois, E.; Calzaferri, G. Structure of nanochannel entrances in stopcock-functionalized zeolite L composites. Angew. Chem. Int. Ed. 2015, 127, 11264–11268. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Verbelen, B.; Dehaen, W. Postfunctionalization of the BODIPY core: Synthesis and spectroscopy. Eur. J. Org. Chem. 2015, 6577–6595. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY dye, the most versatile fluorophore ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Gartzia-Rivero, L.; Baluelos, J.; Izquierdo, U.; Barrio, V.L.; Bizkarra, K.; Cambra, J.F.; López-Arbeloa, I. Microwave Synthesis of LTL Zeolites with Tunable Size and Morphology: An Optimal Support for Metal-Catalyzed Hydrogen Production from Biogas Reforming Processes. Part. Part. Syst. Charact. 2014, 31, 110–120. [Google Scholar] [CrossRef]








| Dyes | Dye Loading a (%) | Dye Ratio | J b (10−13 M−1 cm3) | R0 c (Å) | λab (nm) | λfl (nm) | |
|---|---|---|---|---|---|---|---|
| D1:D2:A | Dmpopop:PM567: Ox1 | 10:10:10 | 1:1:1 | D1-D2 = 1.0 | 55.0 | 364 | 429 |
| D2-A = 1.8 | 65.8 | 518 | 539 | ||||
| 646 | 669 | ||||||
| C165:PM546: Ox4 | 10:10:10 | 1:1:1 | D1-D2 = 1.1 | 59.1 | 360 | 424 | |
| D2-A = 2.0 | 67.0 | 495 | 509 | ||||
| 614 | 638 | ||||||
| 10:10:2 | 1:1:0.2 | D1-D2 = 1.1 | 59.1 | 360 | 424 | ||
| D2-A = 2.0 | 67.0 | 495 | 509 | ||||
| 614 | 638 | ||||||
| D:A | Dmpopop: Stopcock | 10:* | 1:0.05 | 0.06 | 34.4 | 364 | 436 |
| 558 | 599 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gartzia-Rivero, L.; Bañuelos, J.; López-Arbeloa, I. Photoactive Nanomaterials Inspired by Nature: LTL Zeolite Doped with Laser Dyes as Artificial Light Harvesting Systems. Materials 2017, 10, 495. https://doi.org/10.3390/ma10050495
Gartzia-Rivero L, Bañuelos J, López-Arbeloa I. Photoactive Nanomaterials Inspired by Nature: LTL Zeolite Doped with Laser Dyes as Artificial Light Harvesting Systems. Materials. 2017; 10(5):495. https://doi.org/10.3390/ma10050495
Chicago/Turabian StyleGartzia-Rivero, Leire, Jorge Bañuelos, and Iñigo López-Arbeloa. 2017. "Photoactive Nanomaterials Inspired by Nature: LTL Zeolite Doped with Laser Dyes as Artificial Light Harvesting Systems" Materials 10, no. 5: 495. https://doi.org/10.3390/ma10050495
APA StyleGartzia-Rivero, L., Bañuelos, J., & López-Arbeloa, I. (2017). Photoactive Nanomaterials Inspired by Nature: LTL Zeolite Doped with Laser Dyes as Artificial Light Harvesting Systems. Materials, 10(5), 495. https://doi.org/10.3390/ma10050495