Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of SNCM
2.3. Characterization
2.4. Adsorption Studies
3. Results and Discussion
3.1. Characterization of the SNCM
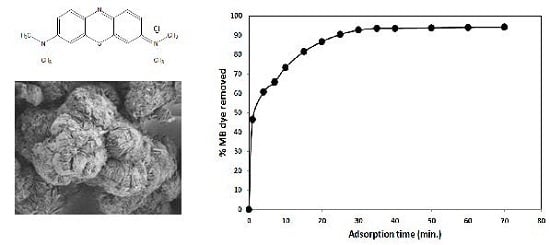
3.2. Effect of Operational Parameters
3.3. Kinetics and Thermodynamics Studies
4. Recycle Study
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, A.; Li, X.; Wang, X. Removal of organic pollutants from reverse osmosis concentrate by electro-fenton process. Adv. Mater. Res. 2014, 2294, 955–959. [Google Scholar] [CrossRef]
- Valero, E.; González-Sánchez, M.-I.; Pérez-Prior, M.-T. Removal of Organic Pollutants from Industrial Wastewater by Treatment with Oxidoreductase Enzymes. In The Handbook of Environmental Chemistry; Springer International Publishing: Berlin, Germany, 2014; pp. 317–339. [Google Scholar]
- Pouran, S.R.; Abdul, A.A.R.; Ashri, W.M.; Daud, W. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al-Harthy, E.A.; Angari, Y.M.; Abdel, S.M. MWCNTs decorated with Mn0.8Zn0.2Fe2O4 nanoparticles for removal of crystal-violet dye from aqueous solutions. Chem. Eng. J. 2014, 255, 156–164. [Google Scholar] [CrossRef]
- Abdel, S.M.; El-Shishtawy, R.M.; Obaid, A.Y. Synthesis of magnetic multi-walled carbon nanotubes/magnetite/chitin magnetic nanocomposite for the removal of Rose Bengal from real and model solution. J. Ind. Eng. Chem. 2014, 20, 3559–3567. [Google Scholar]
- Ahmed, M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, X.; Wang, J.; Lin, P.; Chen, C.; Zhang, X.; Suffet, I.H. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jena, H.M. Adsorption of Cr (VI) from aqueous solution by prepared high surface area activated carbon from Fox nutshell by chemical activation with H3PO4. J. Environ. Chem. Eng. 2017, 5, 2032–2041. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of copper (II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Bazzar, M.; Ramzanian, S.F.; Tajbakhsh, M. Sulfonated nanoclay minerals as a recyclable eco-friendly catalyst for the synthesis of quinoxaline derivatives in green media. Appl. Clay Sci. 2014, 88, 178–185. [Google Scholar] [CrossRef]
- Gómez, L.; Hueso, J.L.; Ortega-Liébana, M.C.; Santamaría, J.; Cronin, S.B. Evaluation of gold-decorated halloysite nanotubes as plasmonicphotocatalysts. Catal. Commun. 2014, 56, 115–118. [Google Scholar] [CrossRef]
- Liu, S.-P. Flame retardant and mechanical properties of polyethylene/magnesium hydroxide/montmorillonite nanocomposites. J. Ind. Eng. Chem. 2014, 20, 2401–2408. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubespolymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Singh, P.; Ghosh, A.K. Torsional, tensile and structural properties of acrylonitrile-butadiene-styrene clay nanocomposites. Mater. Des. 2014, 55, 137–145. [Google Scholar] [CrossRef]
- Maryan, A.S.; Montazer, M. Natural and organo-montmorillonite as antibacterial nanoclays for cotton garment. J. Ind. Eng. Chem. 2015, 22, 164–170. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; ALOthman, Z.A.; Abdel-Khalek, A.A.; Tawfeek, A.M. Characterization of reactive amphiphilic montmorillonite nanogels and its application for removal of toxic cationic dye and heavy metals water pollutants. J. Ind. Eng. Chem. 2015, 31, 374–384. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, L.; Zhu, L.; Guo, J.; Wang, A. Synthesis of covalently crosslinked attapulgite/poly(acrylic acid-co-acrylamide) nanocomposite hydrogels and their evaluation as adsorbent for heavy metal ions. J. Ind. Eng. Chem. 2015, 23, 188–193. [Google Scholar] [CrossRef]
- Maris, S.; Meira, M.; IzéJardim, A.; Brandelli, A. Adsorption of nisin and pediocin on nanoclays. Food Chem. 2015, 188, 161–169. [Google Scholar]
- Shirzad-Siboni, M.; Khataee, A.; Hassani, A.; Karaca, S. Preparation, characterization and application of a CTAB-modified nanoclay for the adsorption of an herbicide from aqueous solutions: Kinetic and equilibrium studies. C. R. Chim. 2015, 18, 204–214. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Pavlova, S.V.; Yakovlev, A.V. Adsorption and in vitro release of vitamin B1 by synthetic nanoclays with montmorillonite structure. Appl. Clay Sci. 2015, 112, 10–16. [Google Scholar] [CrossRef]
- Rasouli, F.; Aber, S.; Salari, D.; Khataee, A.R. Optimized removal of reactive Navy Blue SP-BR by organo-montmorillonite based adsorbents through central composite design. Appl. Clay Sci. 2014, 87, 228–234. [Google Scholar] [CrossRef]
- Loginov, M.; Lebovka, N.; Vorobiev, E. Hybrid multiwalled carbon nanotube—Laponite sorbent for removal of methylene blue from aqueous solutions. J. Colloid Interface Sci. 2014, 431, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Zheng, Y.; Wang, A. Adsorption and release of ofloxacin from acid- and heat-treated halloysite. Colloid Surf. B 2014, 113, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Abdullayev, E.; Vasiliev, A.; Lvov, Y. Halloysitenanotubule clay for efficient water purification. J. Colloid Interface Sci. 2013, 406, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Darvishi, R.; Soltani, C.; Karaca, S.; Khataee, A. Preparation of montmorillonite–alginate nanobiocomposite for adsorption of a textile dye in aqueous phase: Isotherm, kinetic and experimental design approaches. J. Ind. Eng. Chem. 2015, 21, 1197–1207. [Google Scholar] [CrossRef]
- Guerra, D.L.; Pinto, A.A.J.; Souza, A.; Airoldi, C.; Viana, R.R. Kinetic and thermodynamic uranyl (II) adsorption process into modified Na-Magadiite and Na-Kanemite. J. Hazard. Mater. 2009, 166, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Guerraa, D.L.; Pintoa, A.A.; Airoldia, C.; Vianab, R.R. Rapid Communication Adsorption of arsenic (III) into modified lamellar Na-magadiite in aqueous medium thermodynamic of adsorption process. J. Solid State Chem. 2008, 181, 3374–3379. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Macedo, T.R.; Airoldi, C. Sodic and acidic crystalline lamellar magadiite adsorbents for the removal of methylene blue from aqueous solutions: Kinetic and equilibrium studies. Sep. Sci. Technol. 2010, 45, 129–141. [Google Scholar] [CrossRef]
- E-Blaison, C.; Sauzeat, E.; Pelletier, M.; Michot, L.J.; Villieras, F.; Humbert, B. Hydration mechanisms and swelling behavior of Na-magadiite. Chem. Mater. 2001, 13, 1480–1486. [Google Scholar] [CrossRef]
- Sault, A.G.; Martino, A.; Kawola, J.S.; Boespflug, E. Novel Sol-Gel-Based Pt Nanocluster catalysts for propane dehydrogenation. J. Catal. 2000, 191, 474–479. [Google Scholar] [CrossRef]
- Fukuoka, A.; Higashimoto, N.; Sakamoto, Y.; Sasaki, M.; Sugimoto, N.; Inagaki, S.; Fukushima, Y.; Ichikawa, M. Ship-in-bottle synthesis and catalytic performances of platinum carbonyl clusters, nanowires, and nanoparticles in micro- and mesoporous materials. Catal. Today 2001, 66, 23–31. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Romero, F.J. Supramolecular templated synthesis of platinum-supported silica. Chem. Commun. 1999, 10, 873–874. [Google Scholar] [CrossRef]
- Eswaramoorthy, M.; Niwa, S.; Toba, M.; Shimada, H.; Raj, A.; Mizukami, F. The conversion of methane with silica-supported platinum catalysts: The effect of catalyst preparation method and platinum particle size. Catal. Lett. 2001, 71, 55–61. [Google Scholar] [CrossRef]
- Schwieger, W.; Selvam, T.; Gravenhorst, O.; Pfander, N.; Schlogl, R.; Mabande, G.T.P. Intercalation of [Pt(NH3)4]2+ ions into layered sodium silicate magadiite: A useful method to enhance their stabilisation in a highly dispersed state. J. Phys. Chem. Solids 2004, 65, 413–420. [Google Scholar] [CrossRef]
- Paz, G.L.E.; Munsignatti, C.O.; Pastore, H.O. Novel catalyst with layered structure: Metal substituted magadiite. J. Mol. Catal. A Chem. 2016, 422, 43–50. [Google Scholar] [CrossRef]
- Moura, A.O.; Prado, A.G.S. Effect of thermal dehydration and rehydration on Namagadiite structure. J. Colloid Interface Sci. 2009, 330, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2001; pp. 167–171. ISBN 0-201-61091-4. [Google Scholar]
- Beneke, K.; Legaly, G. Kenyaite-synthesis and properties. Am. Mineral. 1983, 68, 818–826. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pieroti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.F.; Rodriguez-Reinoso, R.J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- De Souza e Silva, J.M.; Paul, G.; Bendall, J.; Bisio, C.; Marchese, L.; Pastore, H.O. Novel insights on magadiite disaggregation: A multitechnique study on thermal stability. Phys. Chem. Chem. Phys. 2013, 28, 13434–13445. [Google Scholar] [CrossRef] [PubMed]
- Kallel, F.; Chaari, F.; Bouaziz, F.; Bettaieb, F.; Ghorbel, R.; Chaabouni, S.E. Sorption and desorption characteristics for the removal of a toxic dye, methylene blue from aqueous solution by a low cost agricultural by-product. J. Mol. Liq. 2016, 219, 279–288. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A novel nanohybrid nanofibrous adsorbent for water purification from dye pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef]
- Abdel Salam, M. Synthesis and characterization of novel manganese oxide nanocorals and their application for the removal of methylene blue from aqueous solution. Chem. Eng. J. 2015, 270, 50–57. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Sahraei, R.; Daneshfar, A. Characterization of zinc oxide nanorods loaded on activated carbon as cheap and efficient adsorbent for removal of methylene blue. J. Ind. Eng. Chem. 2105, 21, 986–993. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. J. Ind. Eng. Chem. 2014, 20, 2317–2324. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, R.; Xiang, T.; Zhao, W.-F.; Zhao, C.-S. Preparation, characterization and application of poly(sodium p-styrenesulfonate)/poly(methyl methacrylate) particles. J. Ind. Eng. Chem. 2016, 34, 415–421. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Han, J.I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Alzaydien, A.S. Adsorption of methylene blue from aqueous solution onto a low-cost natural Jordanian Tripoli. Am. J. Environ. Sci. 2009, 5, 1047–1058. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Preferential adsorption behavior of methylene blue dye onto surface hydroxyl group enriched TiO2 nanotube and its photocatalytic regeneration. J. Colloid Interface Sci. 2014, 433, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Gobi, K.; Mashitah, M.D.; Vadivelu, V.M. Adsorptive removal of methylene blue using novel adsorbent from palm oil mill effluent waste activated sludge: Equilibrium, thermodynamics and kinetic studies. Chem. Eng. J. 2011, 171, 1246–1252. [Google Scholar] [CrossRef]











© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, M. Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water. Materials 2017, 10, 760. https://doi.org/10.3390/ma10070760
Mokhtar M. Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water. Materials. 2017; 10(7):760. https://doi.org/10.3390/ma10070760
Chicago/Turabian StyleMokhtar, Mohamed. 2017. "Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water" Materials 10, no. 7: 760. https://doi.org/10.3390/ma10070760
APA StyleMokhtar, M. (2017). Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water. Materials, 10(7), 760. https://doi.org/10.3390/ma10070760