Micro/Nano Structural Tantalum Coating for Enhanced Osteogenic Differentiation of Human Bone Marrow Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
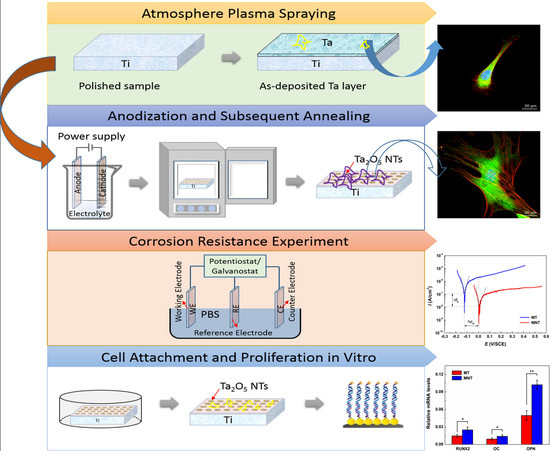
2.1. Preparation of Plasma-Sprayed Tantalum Coating
2.2. Fabrication of Ta2O5 NTs by Anodization
2.3. Characterization of Coatings
2.4. Protein Adsorption Assay
2.5. Cell Morphology
2.6. Cell Cytoskeleton Immunofluorescence
2.7. hBMSCs Adhesion and Proliferation
2.8. Matrix Mineralization
2.9. Osteocalcin Secretion
2.10. Real-Time PCR
2.11. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization
3.2. Electrical Performance
3.3. Protein Adsorption
3.4. Cell Morphology and Cytoskeleton Fluorescence Staining
3.5. Cell Attachment and Proliferation
3.6. Extracellular Matrix Mineralization and Osteocalcin Secretion
3.7. Osteogenesis-Related Genes Expression
4. Conclusions
- (1)
- The Ta2O5 NTs with diameter of about 15 nm are deposited on the micro-porous plasma-sprayed tantalum coating by a two-step anodization technique.
- (2)
- The corrosion resistance of MNT coating has been enhanced by approximately one order of magnitude, because the tantalum oxides covered on the surface can work as a barrier to decrease the release of metal ions to the solution of the SBF.
- (3)
- The MNT coating exhibits better cell spreading of hBMSCs and improved cytocompatibility in vitro. Moreover, it can enhance the hBMSCs differentiation which provides 1.5~2.1 times improvement in gene expression compared with the MT coating.
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical Statement
References
- Wisbey, A.; Gregson, P.J.; Peter, L.M.; Tuke, M. Effect of surface treatment on the dissolution of titanium-based implant materials. Biomaterials 1991, 12, 470–473. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Biomaterials and Tissue Biomechanics: A Match Made in Heaven? Materials 2017, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.; Walls, M.; Rondot, B.; Da Cunha Belo, M.; Guidoin, R. Electrochemical and microstructural studies of tantalum and its oxide films for biomedical applications in endovascular surgery. J. Mater. Sci. Mater. Med. 2002, 13, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Sista, S.; Wen, C.E.; Hodgson, P.D.; Pande, G. The influence of surface energy of titanium-zirconium alloy on osteoblast cell functions in vitro. J. Biomed. Mater. Res. Part A 2011, 97, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J. Nanostructured surfaces: Cell engineering and cell biology. Nanomedicine 2009, 4, 247. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, C.J.; Brammer, K.S.; Noh, K.; Johnston, G.; Jin, S. Tantalum coating on TiO2 nanotubes induces superior rate of matrix mineralization and osteofunctionality in human osteoblasts. Mater. Sci. Eng. C 2014, 37, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.G.; Hill, E.W.; Bayat, A. Designing implant surface topography for improved biocompatibility. Expert Rev. Med. Devices 2013, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nishiguchi, S.; Nakamura, T. Bioactive macroporous titanium surface layer on titanium substrate. J. Biomed. Mater. Res. Part A 2000, 52, 553–557. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Ji, H.; Ding, C. Effect of Ti–OH formation on bioactivity of vacuum plasma sprayed titanium coating after chemical treatment. Surface Coat. Technol. 2007, 202, 494–498. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R. Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, Y.; Yang, F.; Huang, Y.; Wang, C.; Dai, K. Porous tantalum coatings prepared by vacuum plasma spraying enhance BSMCs osteogenic differentiation and bone regeneration in vitro and in vivo. PLoS ONE 2013, 8, e66263. [Google Scholar]
- Ding, D.; Xie, Y.; Li, K.; Zheng, X. Improved Mechanical Compatibility and Cytocompatibility of Ta/Ti Double–Layered Composite Coating. J. Therm. Spray Technol. 2017, 26, 1292–1300. [Google Scholar] [CrossRef]
- Kriparamanan, R.; Aswath, P.; Zhou, A.; Tang, L.; Nguyen, K.T. Nanotopography: Cellular responses to nanostructured materials. J. Nanosci. Nanotechnol. 2006, 6, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Vaahtio, M.; Peltola, T.; Hentunen, T.; Yleänen, H.; Areva, S.; Wolke, J.; Salonen, J.I. The properties of biomimetically processed calcium phosphate on bioactive ceramics and their response on bone cells. J. Mater. Sci. Mater. Med. 2006, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Areva, S.; Paldan, H.; Peltola, T.; Neärhi, T.; Jokinen, M.; Lindaén, M. Use of sol-gel-derived titania coating for direct soft tissue attachment. J. Biomed. Mater. Res. Part A 2004, 70, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Hansen, J.C.; Siedlecki, C.A.; Runt, J.; Donahue, H.J. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J. R. Soc. Interface 2005, 2, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; McCloy, D.; Robertson, M.; Wilkinson, C.D.; Oreffo, R.O. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials 2006, 27, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zheng, X.; Huang, L.; Ding, C. Influence of hierarchical hybrid micro/nano-structured surface on biological performance of titanium coating. J. Mater. Sci. 2012, 47, 1411–1417. [Google Scholar] [CrossRef]
- Xie, Y.; Ao, H.; Xin, S.; Zheng, X.; Ding, C. Enhanced cellular responses to titanium coating with hierarchical hybrid structure. Mater. Sci. Eng. C 2014, 38, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.S.; Kim, S.; Kim, H.E.; Jang, T.S. Hierarchical micro-nano structured Ti6Al4V surface topography via two–step etching process for enhanced hydrophilicity and osteoblastic responses. Mater. Sci. Eng. C 2017, 73, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.I.; Abrams, G.A.; Bertics, P.J.; Murphy, C.J.; Nealey, P.F. Epithelial contact guidance on well-defined micro-and nanostructured substrates. J. Cell Sci. 2003, 116, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, S.; Shen, M.; Cheng, B.; Li, Y.; Liu, X.; Kong, L. Osteogenic activity of titanium surfaces with hierarchical micro-/nano-structures obtained by hydrofluoric acid treatment. Int. J. Nanomed. 2017, 12, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, H.; Wang, J.; Chen, S.; Ma, Y.; Zhang, Z. Study on the anticorrosion, biocompatibility, and osteoinductivity of tantalum decorated with tantalum oxide nanotube array films. ACS Appl. Mater. Interfaces 2012, 4, 4516–4523. [Google Scholar] [CrossRef] [PubMed]
- Ruckh, T.; Porter, J.R.; Allam, N.K.; Feng, X.; Grimes, C.A.; Popat, K.C. Nanostructured tantala as a template for enhanced osseointegration. Nanotechnology 2008, 20, 045102. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Haïat, G.; Wang, H.L.; Brunski, J. Effects of biomechanical properties of the bone-implant interface on dental implant stability: From in silico approaches to the patient’s mouth. Annu. Rev. Biomed. Eng. 2014, 16, 187–213. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Zhao, L.; Mei, S.; Chu, P.K.; Zhang, Y.; Wu, Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, X.; Barbieri, D.; Barradas, A.M.; de Bruijn, J.D.; Van Blitterswijk, C.A.; Yuan, H. The size of surface microstructures as an osteogenic factor in calcium phosphate ceramics. Acta Biomater. 2014, 10, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xie, Y.; Li, K.; Hu, D.; Zhao, J.; Zheng, X.; Tang, T. Rock–regulated synergistic effect of macropore/nanowire topography on cytoskeletal distribution and cell differentiation. RSC Adv. 2015, 5, 101834–101842. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.N.; Anitha, V.C.; Joo, S.W. Improved electrochemical properties of morphology-controlled titania/titanate nanostructures prepared by in-situ hydrothermal surface modification of self-source Ti substrate for high-performance supercapacitors. Sci. Rep. 2017, 7, 13227. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Razak, B.A.; Dabbagh, A.; Nasiri-Tabrizi, B.; Kasim, N.H.A.; Basirun, W.J. Optimizing PVD conditions for electrochemical anodization growth of well-adherent Ta2O5 nanotubes on Ti–6Al–4V alloy. RSC Adv. 2016, 6, 78999–79015. [Google Scholar] [CrossRef]
- Mendonca, G.; Mendonca, D.B.; Aragao, F.J.; Cooper, L.F. Advancing dental implant surface technology from micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Razak, B.A.; Nasiri-Tabrizi, B.; Dabbagh, A.; Kasim, N.H.A.; Basirun, W.J.; Sulaiman, E.B. Nanomechanical properties, wear resistance and in–vitro characterization of Ta2O5 nanotubes coating on biomedical grade Ti–6Al–4V. J. Mech. Behav. Biomed. Mater. 2017, 66, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Frant, M.; Bossert, J.; Hildebrandb, G.; Liefeithb, K.; Jandt, K.D. Surface functionalized titanium thin films: Zeta-potential, protein adsorption and cell proliferation. Colloids Surf. B Biointerfaces 2006, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jenney, C.R.; Anderson, J.M. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J. Biomed. Mater. Res. Part A 2000, 49, 435–447. [Google Scholar] [CrossRef]
- Tsai, J.A.; Lagumdzija, A.; Stark, A.; Kindmark, H. Albumin-bound lipids induce free cytoplasmic calcium oscillations in human osteoblast-like cells. Cell Biochem. Funct. 2007, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamaguchi, M. Role of albumin in osteoblastic cells: Enhancement of cell proliferation and suppression of alkaline phosphatase activity. Int. J. Mol. Med. 2004, 14, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.A.; Reyes, C.D.; Burns, K.L.; García, A.J. Simple application of fibronectin–mimetic coating enhances osseointegration of titanium implants. J. Cell. Mol. Med. 2009, 13, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Schadler, L.S.; Siegel, R.W.; Bizios, R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001, 7, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Veeverslowe, J.; Ball, S.G.; Shuttleworth, A.; Kielty, C.M. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J. Cell Sci. 2011, 124, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Chang, J.H.; Huang, H.H. Corrosion resistance and biocompatibility of titanium surface coated with amorphous tantalum pentoxide. Thin Solid Films 2013, 528, 130–135. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, L.; Xing, L.; Chen, D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 2010, 28, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Sun, Q.; Zhang, J.; Ge, S.; Li, S.; Zhao, Y.; Yang, P. Multilineage differentiation of dental follicle cells and the roles of Runx2 over-expression in enhancing osteoblast/cementoblast-related gene expression in dental follicle cells. Cell Prolif. 2010, 43, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shen, X.; Hu, Y.; Ma, P.; Cai, K. Fabrication of tantalum oxide layers onto titanium substrates for improved corrosion resistance and cytocompatibility. Surface Coat. Technol. 2015, 272, 58–65. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Zhang, T.L.; Wang, K. Alterations of ovariectomized rat bone and impact of non–collagenous proteins on mineralization. Jt. Bone Spine 2009, 76, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zurick, K.M.; Qin, C.; Bernards, M.T. Mineralization induction effects of osteopontin, bone sialoprotein, and dentin phosphoprotein on a biomimetic collagen substrate. J. Biomed. Mater. Res. Part A 2013, 101, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Sakamoto, K.; Nagaoka, I. Effect of glucosamine, a therapeutic agent for osteoarthritis, on osteoblastic cell differentiation. Int. J. Mol. Med. 2011, 28, 373–379. [Google Scholar] [PubMed]














| Target Gene | Direction | 5′-3′ Primer Sequence |
|---|---|---|
| β-actin | F | 5′-CATGTACGTTGCTATCCAGGC-3′ |
| R | 5′-CTCCTTAATGTCACGCACGAT-3′ | |
| Runx2 | F | 5′-TGGTTACTGTCATGGCGGGTA-3′ |
| R | 5′-TCTCAGATCGTTGAACCTTGCTA-3′ | |
| OC | F | 5′-CACTCCTCGCCCTATTGGC-3′ |
| R | 5′-CCCTCCTGCTTGGACACAAAG-3′ | |
| OPN | F | 5′-CTCCATTGACTCGAACGACTC-3′ |
| R | 5′-CAGGTCTGCGAAACTTCTTAGAT-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, D.; Xie, Y.; Li, K.; Huang, L.; Zheng, X. Micro/Nano Structural Tantalum Coating for Enhanced Osteogenic Differentiation of Human Bone Marrow Stem Cells. Materials 2018, 11, 546. https://doi.org/10.3390/ma11040546
Ding D, Xie Y, Li K, Huang L, Zheng X. Micro/Nano Structural Tantalum Coating for Enhanced Osteogenic Differentiation of Human Bone Marrow Stem Cells. Materials. 2018; 11(4):546. https://doi.org/10.3390/ma11040546
Chicago/Turabian StyleDing, Ding, Youtao Xie, Kai Li, Liping Huang, and Xuebin Zheng. 2018. "Micro/Nano Structural Tantalum Coating for Enhanced Osteogenic Differentiation of Human Bone Marrow Stem Cells" Materials 11, no. 4: 546. https://doi.org/10.3390/ma11040546
APA StyleDing, D., Xie, Y., Li, K., Huang, L., & Zheng, X. (2018). Micro/Nano Structural Tantalum Coating for Enhanced Osteogenic Differentiation of Human Bone Marrow Stem Cells. Materials, 11(4), 546. https://doi.org/10.3390/ma11040546