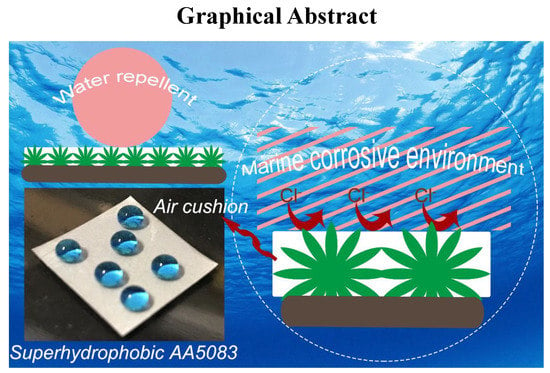
Lotus-Inspired Multiscale Superhydrophobic AA5083 Resisting Surface Contamination and Marine Corrosion Attack
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation of Superhydrophobic AA5083
2.3. Characterization
2.4. Electrochemical Test
3. Results and Discussion
3.1. Surface Morphology and Wettability Behavior
3.2. Low Surface Adhesivity
3.3. Chemical Composition
3.4. Self-Cleaning Ability and NaCl Self-Propelling
3.5. Marine Corrosion Protection
3.6. Long-Term Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Mills, R.J.; Lattimer, B.Y.; Case, S.W.; Mouritz, A.P. The influence of sensitization and corrosion on creep of 5083-H116. Corros. Sci. 2018, 143, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Tao, Y.; Han, X.; Cui, C. Enhanced corrosion resistance of 5083 aluminum alloy by refining with nano-CeB6/Al inoculant. Appl. Surf. Sci. 2019, 484, 403–408. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yun, F.F.; Wang, Y.; Yao, L.; Dou, S.; Liu, K.; Jiang, L.; Wang, X. Desert beetle-inspired superwettable patterned surfaces for water harvesting. Small 2017, 13, 1701403. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Gao, X.F.; Jiang, L. Biophysics: Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.; Hou, B. One-step electrodeposition fabrication of a superhydrophobic surface on an aluminum substrate with enhanced self-cleaning and anticorrosion properties. RSC Adv. 2015, 5, 100000–100010. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, X.; Li, Y.; Hou, B. Fabrication of durable anticorrosion superhydrophobic surfaces on aluminum substrates via a facile one-step electrodeposition approach. RSC Adv. 2016, 6, 35455–35465. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Maurya, R.; Balani, K. Superhydrophobic self-floating carbon nanofiber coating for efficient gravity-directed oil/water separation. J. Mater. Chem. A 2017, 5, 2936–2946. [Google Scholar] [CrossRef]
- Beshkar, F.; Khojasteh, H.; Salavati-Niasari, M. Recyclable magnetic superhydrophobic straw soot sponge for highly efficient oil/water separation. J. Colloid Interface Sci. 2017, 497, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.B.; Patir, A.; Page, K.; Lu, Y.; Allan, E.; Parkin, I.P. Buoyancy increase and drag-reduction through a simple superhydrophobic coating. Nanoscale 2017, 9, 7588–7594. [Google Scholar] [CrossRef] [Green Version]
- Taghvaei, E.; Moosavi, A.; Nouri-Borujerdi, A.; Daeian, M.A.; Vafaeinejad, S. Superhydrophobic surfaces with a dual-layer micro- and nanoparticle coating for drag reduction. Energy 2017, 125, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Jin, J.; Liu, J.; Yan, Y.; Han, Z.; Ren, L. Anti-icing property of bio-inspired micro-structure superhydrophobic surfaces and heat transfer model. Appl. Surf. Sci. 2017, 400, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Liao, R.; Zhao, X.; Song, X.; Qiao, Z.; Guo, C.; Zhuang, A.; Yuan, Y. Anti-frosting performance of superhydrophobic surface with ZnO nanorods. Appl. Therm. Eng. 2017, 110, 39–48. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zheng, X.; Chen, Z.; Zhou, Q.; Chen, Y. 3D-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Adv. Mater. 2018, 30, 1704912. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Jiao, W.; Wang, R.; Niu, Y.; Hao, L.; Yang, F.; Liu, W. A biomimetic, multifunctional, superhydrophobic graphene film with self-sensing and fast recovery properties for microdroplet transportation. J. Mater. Chem. A 2017, 5, 17325–17334. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Q.; Zhang, H.; Wang, C.; Wang, L.; Xiang, B.; Fan, Y.; Guo, C.F.; Ruan, S. Laser direct writing of tree-shaped hierarchical cones on a superhydrophobic film for high-efficiency water collection. ACS Appl. Mater. Interfaces 2017, 9, 29248–29254. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Lee, C.; Nam, Y. Influence of geometric patterns of microstructured superhydrophobic surfaces on water-harvesting performance via dewing. Langmuir 2014, 30, 15468–15476. [Google Scholar] [CrossRef] [PubMed]
- Arukalam, I.O.; Oguzie, E.E.; Li, Y. Nanostructured superhydrophobic polysiloxane coating for high barrier and anticorrosion applications in marine environment. J. Colloid Interface Sci. 2018, 512, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, W.; Zhu, Q.; Li, Y.; Hou, B. Ultrafast one step construction of non-fluorinated superhydrophobic aluminum surfaces with remarkable improvement of corrosion resistance and anti-contamination. J. Colloid Interface Sci. 2018, 532, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A. Silicone/graphene oxide sheet-alumina nanorob ternary composite for superhydrophobic antifouling coating. Prog. Org. Coat. 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhao, X.; Hu, X.; Yang, L.; Wang, N.; Li, Y.; Hou, B. Biomimetic one step fabrication of manganese stearate superhydrophobic surface as an efficient barrier against marine corrosion and Chlorella vulgaris-induced biofouling. Chem. Eng. J. 2016, 306, 441–451. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Chen, H.; Chen, D. Superhydrophobic behavior achieved from hydrophilic surfaces. Appl. Phys. Lett. 2009, 95, 084104. [Google Scholar] [CrossRef]
- Hoshian, S.; Jokinen, V.; Somerkivi, V.; Lokanathan, A.R.; Franssila, S. Robust superhydrophobic silicon without a low surface-energy hydrophobic coating. ACS Appl. Mater. Interfaces 2015, 7, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of superhydrophobic aluminum surfaces with novel micro-terrace nano-leaf hierarchical structure. Appl. Surf. Sci. 2018, 451, 207–217. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Hybrid laser ablation and chemical modification for fast fabrication of bio-inspired super-hydrophobic surface with excellent self-cleaning, stability and corrosion resistance. J. Bionic Eng. 2019, 16, 13–26. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Mo, J.; Sun, R.; Li, Z.; Guo, Z. Fabrication of superhydrophobic aluminum surface by droplet etching and chemical modification. Colloid Surf. A 2019, 567, 205–212. [Google Scholar] [CrossRef]
- Wang, G.; Shen, Y.; Tao, J.; Luo, X.; Jin, M.; Xie, Y.; Li, Z.; Guo, S. Facilely constructing micro-nanostructure superhydrophobic aluminum surface with robust ice-phobicity and corrosion resistance. Surf. Coat. Technol. 2017, 329, 224–231. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of functional nanoengineering and nanosecond laser texturing for design of superhydrophobic aluminum alloy with exceptional mechanical and chemical properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, K.A.; Domantovsky, A.G. Laser tailoring the surface chemistry and morphology for wear, scale and corrosion resistance superhydrophobic coatings. Langmuir 2018, 34, 7059–7066. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, Z. Insitu growth of durable superhydrophobic Mg-Al layered double hydroxides nanoplatelets on aluminum alloys for corrosion resistance. J. Alloys Comp. 2018, 767, 382–391. [Google Scholar] [CrossRef]
- Zhang, B.; Guan, F.; Zhao, X.; Zhang, Y.; Li, Y.; Duan, J.; Hou, B. Micro-nano textured superhydrophobic 5083 aluminum alloy as a barrier against marine corrosion and sulfate-reducing bacteria adhesion. J. Taiwan Inst. Chem. Eng. 2019, 97, 433–440. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Zhu, Q.; Wang, X.; Zhao, X.; Sun, C.; Li, Y.; Hou, B. Controllable Dianthus caryophyllus-like superhydrophilic/superhydrophobic hierarchical structure based on self-congregated nanowires for corrosion inhibition and biofouling mitigation. Chem. Eng. J. 2017, 312, 317–327. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Z.; Zhang, B.; Zhai, X.; Liu, S.; Sun, X.; Zhu, Q.; Hou, B. Effect of graphene oxide on anticorrosion performance of polyelectrolyte multilayer for 2A12 aluminum alloy substrates. RSC Adv. 2017, 7, 33764–33774. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhang, B.; Jin, Z.; Chen, C.; Zhu, Q.; Hou, B. Epoxy coating modified by 2D MoS2/SDBS: Fabrication, anticorrosion behavior and inhibition mechanism. RSC Adv. 2016, 6, 97512–97522. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Q.; Li, Y.; Hou, B. Facile fluorine-free one step fabrication of superhydrophobic aluminum surface towards self-cleaning and marine anticorrosion. Chem. Eng. J. 2018, 352, 625–633. [Google Scholar] [CrossRef]










| Parameters | Specimens | |
|---|---|---|
| Pristine AA5083 | Superhydrophobic AA5083 | |
| Rs (Ω·cm2) | 2.43 | 4.09 |
| Qfilm (Ω−1·cm−2·sn) | / | 1.19 × 10−10 |
| n1 | / | 1 |
| Rfilm (Ω·cm2) | / | 8.24 × 107 |
| Qoxide (Ω−1·cm−2·sn) | 5.84 × 10−9 | 1.66 × 10−9 |
| n2 | 0.79 | 0.59 |
| Roxide (Ω·cm2) | 1.47 × 104 | 8.29 × 107 |
| Qdl (Ω−1·cm−2·sn) | 6.77 × 10−6 | 3.54 × 10−10 |
| n3 | 0.44 | 0.80 |
| Rct (Ω·cm2) | 4.44 × 104 | 1.14 × 106 |
| η (%) | / | 96.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Xu, W.; Zhu, Q.; Yuan, S.; Li, Y. Lotus-Inspired Multiscale Superhydrophobic AA5083 Resisting Surface Contamination and Marine Corrosion Attack. Materials 2019, 12, 1592. https://doi.org/10.3390/ma12101592
Zhang B, Xu W, Zhu Q, Yuan S, Li Y. Lotus-Inspired Multiscale Superhydrophobic AA5083 Resisting Surface Contamination and Marine Corrosion Attack. Materials. 2019; 12(10):1592. https://doi.org/10.3390/ma12101592
Chicago/Turabian StyleZhang, Binbin, Weichen Xu, Qingjun Zhu, Shuai Yuan, and Yantao Li. 2019. "Lotus-Inspired Multiscale Superhydrophobic AA5083 Resisting Surface Contamination and Marine Corrosion Attack" Materials 12, no. 10: 1592. https://doi.org/10.3390/ma12101592
APA StyleZhang, B., Xu, W., Zhu, Q., Yuan, S., & Li, Y. (2019). Lotus-Inspired Multiscale Superhydrophobic AA5083 Resisting Surface Contamination and Marine Corrosion Attack. Materials, 12(10), 1592. https://doi.org/10.3390/ma12101592