Synthesis of Structurally Precise Polysiloxanes via the Piers–Rubinsztajn Reaction
Abstract
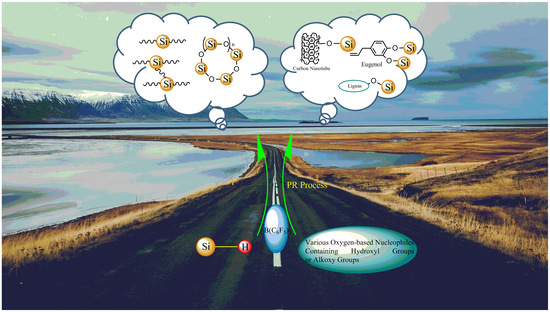
:1. Introduction
2. Advantages of the PR Reaction over Traditional Synthetic Methods
3. Morphology-Controlled Synthesis of Polysiloxanes via the PR Reaction
3.1. Synthesis of Linear Polysiloxanes
3.2. Synthesis of Cyclic Polysiloxanes
3.3. Synthesis of Bridge-link-type Polysiloxanes
4. Synthesis of Biomass-Structure Polysiloxanes via the PR Reaction
4.1. Synthesis of Lignin-Silicone Composites
4.2. Synthesis of Eugenol-silicone Composites
5. Synthesis of Inorganoparticle-Silicone Polymers via the PR Reaction
5.1. Synthesis of Carbon Nanotube-silicone Materials
5.2. Synthesis of Graphene Oxide-Silicone Materials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brook, M.A. Silicon in Organic, Organometallic, and Polymer Chemistry; Wiley-Interscience: New York, NY, USA, 2000; p. 308. ISBN 0-471-19658-4. [Google Scholar]
- Brook, M.A.; Grande, J.B.; Ganachaud, F. New synthetic strategies for structured silicones using b(c6f5)(3). Adv. Polym. Sci. 2010, 235, 161–183. [Google Scholar]
- Wakabayashi, R.; Kuroda, K. Siloxane-bond formation promoted by lewis acids: A nonhydrolytic sol-gel process and the piers-rubinsztajn reaction. ChemPlusChem 2013, 78, 764–774. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science, the Physics and Chemistry of Sol-Gel Processing; Academic Press: New York, NY, USA, 1990; p. 908. ISBN 0-12-134970-5. [Google Scholar]
- Fuchise, K.; Igarashi, M.; Sato, K.; Shimada, S. Organocatalytic controlled/living ring-opening polymerization of cyclotrisiloxanes initiated by water with strong organic base catalysts. J. Chem. Sci. 2018, 9, 2879–2891. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, K.; Shimada, S.; Sato, K. Sequence-controlled catalytic one-pot synthesis of siloxane oligomers. Chem. Eur. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.A. New control over silicone synthesis using Si–H chemistry: The piers rubinsztajn reaction. Chem. Eur. J. 2018. [Google Scholar] [CrossRef]
- Chauvier, C.; Thuéry, P.; Cantat, T. Silyl formates as surrogates of hydrosilanes and their application in the transfer hydrosilylation of aldehydes. Angew. Chem. Int. Ed. 2016, 55, 14096–14100. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, V.V.; Rubin, M.; Benson, S.; Liu, J.X.; Yamamoto, Y. A novel B(C(6)F(5))3-catalyzed reduction of alcohols and cleavage of aryl and alkyl ethers with hydrosilanes. J. Org. Chem. 2000, 65, 6179–6186. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.J.; Blackwell, J.M.; Piers, W.E. Studies on the mechanism of B(C(6)F(5))3-catalyzed hydrosilation of carbonyl functions. J. Org. Chem. 2000, 65, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztajn, S.; Cella, J.A. A new polycondensation process for the preparation of polysiloxane copolymers. Macromolecules 2005, 38, 1061–1063. [Google Scholar] [CrossRef]
- Szawiola, A.M.; de Melo Souza, N.; Lessard, B.H.; Bender, T.P. Phenoxylated siloxane-based polymers via the piers-rubinsztajn process. Polym. Int. 2017, 66, 1324–1328. [Google Scholar] [CrossRef]
- Madsen, F.B.; Javakhishvili, I.; Jensen, R.E.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. Synthesis of telechelic vinyl/allyl functional siloxane copolymers with structural control. Polym. Chem. 2014, 5, 7054–7061. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kawakami, Y. Efficient synthesis of poly(silyl ether)s by pd/c and rhcl(pph3)3-catalyzed cross-dehydrocoupling polymerization of bis(hydrosilane)s with diols. Macromolecules 1999, 32, 6871–6873. [Google Scholar] [CrossRef]
- Zhang, R.; Mark, J.E.; Pinhas, A.R. Dehydrocoupling polymerization of bis-silanes and disilanols to poly(silphenylenesiloxane) as catalyzed by rhodium complexes. Macromolecules 2000, 33, 3508–3510. [Google Scholar] [CrossRef]
- Li, Y.; Seino, M.; Kawakami, Y. Asymmetric synthesis of optically active poly(silyl ether)s having reactive si−h groups by stereoselective cross-dehydrocoupling polymerization of bis(silane)s with diols. Macromolecules 2000, 33, 5311–5314. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Concepcion, P.; Garcia, H. Dehydrogenative coupling of silanes with alcohols catalyzed by cu3(btc)2. Chem. Commun. 2016, 52, 2725–2728. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Kubo, K.; Matsumoto, T.; Sato, K.; Ando, W.; Shimada, S. Pd/c-catalyzed cross-coupling reaction of benzyloxysilanes with halosilanes for selective synthesis of unsymmetrical siloxanes. RSC Adv. 2014, 4, 19099–19102. [Google Scholar] [CrossRef]
- Houghton, A.Y.; Hurmalainen, J.; Mansikkamäki, A.; Piers, W.E.; Tuononen, H.M. Direct observation of a borane-silane complex involved in frustrated lewis-pair-mediated hydrosilylations. Nat. Chem. 2014, 6, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.B.; Urlich, T.; Dickie, T.; Brook, M.A. Silicone dendrons and dendrimers from orthogonal sih coupling reactions. Polym. Chem. 2014, 5, 6728–6739. [Google Scholar] [CrossRef]
- Dudziec, B.; Rzonsowska, M.; Marciniec, B.; Brząkalski, D.; Woźniak, B. New mono- and diethynylsiloxysilsesquioxanes—Efficient procedures for their synthesis. Dalton Trans. 2014, 43, 13201–13207. [Google Scholar] [CrossRef]
- Sodkhomkhum, R.; Ervithayasuporn, V. Synthesis of poly(siloxane/double-decker silsesquioxane) via dehydrocarbonative condensation reaction and its functionalization. Polymer 2016, 86, 113–119. [Google Scholar] [CrossRef]
- Wu, C.-B.; Jin, Y.-H.; Li, W.; Gao, D.-H.; Jia, M.-Q. Synthesis and characterization of a silicone resin with silphenylene units in si–o–si backbones II. High Perform. Polym. 2010, 22, 959–973. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, L.; Diao, S.; Feng, S.; Zhang, C. Study on the synthesis and thermal degradation of silicone resin containing silphenylene units. Thermochim. Acta 2011, 521, 170–175. [Google Scholar] [CrossRef]
- Yang, Z.; Han, S.; Zhang, R.; Feng, S.; Zhang, C.; Zhang, S. Effects of silphenylene units on the thermal stability of silicone resins. Polym. Degrad. Stab. 2011, 96, 2145–2151. [Google Scholar] [CrossRef]
- Tian, S.; Li, J.; Cai, Z.; Shi, B.; Chen, W.; Cheng, Y. Piers-rubinsztajn reaction for bcb functionalized silphenylene/silbiphenylene siloxane oligomers to highly crosslinked low-k thermosets. Eur. Polym. J. 2018, 108, 373–379. [Google Scholar] [CrossRef]
- Polymeropoulos, G.; Bilalis, P.; Hadjichristidis, N. Well-defined cyclic triblock terpolymers: A missing piece of the morphology puzzle. ACS Macro Lett. 2016, 5, 1242–1246. [Google Scholar] [CrossRef]
- Morgese, G.; Trachsel, L.; Romio, M.; Divandari, M.; Ramakrishna, S.N.; Benetti, E.M. Cover picture: Topological polymer chemistry enters surface science: Linear versus cyclic polymer brushes. Angew. Chem. Int. Ed. 2016, 55, 15445. [Google Scholar] [CrossRef]
- Chen, N.; Reeja-Jayan, B.; Liu, A.; Lau, J.; Dunn, B.; Gleason, K.K. Icvd cyclic polysiloxane and polysilazane as nanoscale thin-film electrolyte: Synthesis and properties. Macromol. Rapid Commun. 2016, 37, 446–452. [Google Scholar] [CrossRef]
- Schmolke, W.; Perner, N.; Seiffert, S. Dynamically cross-linked polydimethylsiloxane networks with ambient-temperature self-healing. Macromolecules 2015, 48, 8781–8788. [Google Scholar] [CrossRef]
- Wu, C.; Yu, J.; Li, Q.; Liu, Y. High molecular weight cyclic polysiloxanes from organocatalytic zwitterionic polymerization of constrained spirocyclosiloxanes. Polym. Chem. 2017, 8, 7301–7306. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y. Cyclic polysiloxanes with linked cyclotetrasiloxane subunits. Angew. Chem. Int. Ed. 2017, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Chen, X.; Wu, S.; Ge, J.; Zhou, X.; Yin, G. Fabrication of reactive poly(phenyl-substituted siloxanes/silsesquioxanes) with Si–H and alkoxy functional groups via the piers–rubinsztajn reaction. Polymers 2018, 10, 1006. [Google Scholar] [CrossRef]
- Macphail, B.; Brook, M.A. Controlling silicone-saccharide interfaces: Greening silicones. Green Chem. 2017, 19, 4373–4379. [Google Scholar] [CrossRef]
- Graiver, D.; Farminer, K.W.; Narayan, R. A review of the fate and effects of silicones in the environment. J. Polym. Environ. 2003, 11, 129–136. [Google Scholar] [CrossRef]
- Zhang, J.F.; Chen, Y.; Brook, M.A. Reductive degradation of lignin and model compounds by hydrosilanes. ACS Sustain. Chem. Eng. 2014, 2, 1983–1991. [Google Scholar] [CrossRef]
- Zhang, J.F.; Chen, Y.; Sewell, P.; Brook, M.A. Utilization of softwood lignin as both crosslinker and reinforcing agent in silicone elastomers. Green Chem. 2015, 17, 1811–1819. [Google Scholar] [CrossRef]
- Leonowicz, A.; Matuszewska, A.; Luterek, J.; Ziegenhagen, D.; Wojtaś-Wasilewska, M.; Cho, N.-S.; Hofrichter, M.; Rogalski, J. Biodegradation of lignin by white rot fungi. Fungal Genet. Biol. 1999, 27, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.J.A.; de Jong, E.; Guran, B.; Abächerli, A. Co-ordination network for lignin—Standardisation, production and applications adapted to market requirements (eurolignin). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Prida, A.; Puech, J.-L. Influence of geographical origin and botanical species on the content of extractives in american, french, and east european oak woods. J. Agric. Food Chem. 2006, 54, 8115–8126. [Google Scholar] [CrossRef]
- Laengert, S.E.; Schneider, A.F.; Chen, Y.; Brook, M.A. Sequential functionalization of a natural crosslinker leads to designer silicone networks. Chem. Asian J. 2017, 12, 1208–1212. [Google Scholar] [CrossRef]
- Tao, Y.; He, F.; Jin, K.; Wang, J.; Wang, Y.; Zhou, J.; Sun, J.; Fang, Q. Facile conversion of plant oil (anethole) to a high-performance material. Polym. Chem. 2017, 8, 2010–2015. [Google Scholar] [CrossRef]
- He, F.; Jin, K.; Wang, Y.; Wang, J.; Zhou, J.; Sun, J.; Fang, Q. High performance polymer derived from a biorenewable plant oil (anethole). ACS Sustain. Chem. Eng. 2017, 5, 2578–2584. [Google Scholar] [CrossRef]
- He, F.; Gao, Y.; Jin, K.; Wang, J.; Sun, J.; Fang, Q. Conversion of a biorenewable plant oil (anethole) to a new fluoropolymer with both low dielectric constant and low water uptake. ACS Sustain. Chem. Eng. 2016, 4, 4451–4456. [Google Scholar] [CrossRef]
- Chen, X.; Fang, L.; Chen, X.; Zhou, J.; Wang, J.; Sun, J.; Fang, Q. A low-dielectric polymer derived from a biorenewable phenol (eugenol). ACS Sustain. Chem. Eng. 2018, 6, 13518–13523. [Google Scholar] [CrossRef]
- Tour, J.; Hudson, J.; Kirshnamoorti, R.; Yurekli, K.; Mitchell, C. Elastomers Reinforced with Carbon Nanotubes. U.S. Patent Application US20070259994A1, 8 November 2007. [Google Scholar]
- Lee, C.; Jug, L.; Meng, E. High strain biocompatible polydimethylsiloxane-based conductive graphene and multiwalled carbon nanotube nanocomposite strain sensors. Appl. Phys. Lett. 2013, 102, 183511. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, B.; He, Y.; Oh, S.; Yu, Y.; Kim, J. Addition of silane-functionalized carbon nanotubes for improved rate capability of LiNi1/3Co1/3Mn1/3O2 cathodes for lithium ion batteries. J. Electrochem. Soc. 2012, 159, A2024–A2028. [Google Scholar] [CrossRef]
- Sim, L.C.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.N.; Goh, T.J. Thermal characterization of Al2O3 and zno reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 430, 155–165. [Google Scholar] [CrossRef]
- Brochu, P.; Stoyanov, H.; Chang, R.; Niu, X.; Hu, W.; Pei, Q. Capacitive energy harvesting using highly stretchable silicone–carbon nanotube composite electrodes. Adv. Energy Mater. 2014, 4, 1300659. [Google Scholar] [CrossRef]
- Cai, L.; Song, L.; Luan, P.; Zhang, Q.; Zhang, N.; Gao, Q.; Zhao, D.; Zhang, X.; Tu, M.; Yang, F.; et al. Super-stretchable, transparent carbon nanotube-based capacitive strain sensors for human motion detection. Sci. Rep. 2013, 3, 3048. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef]
- Chadwick, R.C.; Grande, J.B.; Brook, M.A.; Adronov, A. Functionalization of single-walled carbon nanotubes via the piers-rubinsztajn reaction. Macromolecules 2014, 47, 6527–6530. [Google Scholar] [CrossRef]
- Vennerberg, D.; Rueger, Z.; Kessler, M.R. Effect of silane structure on the properties of silanized multiwalled carbon nanotube-epoxy nanocomposites. Polymer 2014, 55, 1854–1865. [Google Scholar] [CrossRef]
- Avilés, F.; Sierra-Chi, C.A.; Nistal, A.; May-Pat, A.; Rubio, F.; Rubio, J. Influence of silane concentration on the silanization of multiwall carbon nanotubes. Carbon 2013, 57, 520–529. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.-P.; Wang, P.; Yu, S.-H. Stretchable and self-healing graphene oxide–polymer composite hydrogels: A dual-network design. Chem. Mater. 2013, 25, 3357–3362. [Google Scholar] [CrossRef]
- Abdullah, S.I.; Ansari, M.N.M. Mechanical properties of graphene oxide (go)/epoxy composites. HBRC J. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Cano, M.; Khan, U.; Sainsbury, T.; O’Neill, A.; Wang, Z.; McGovern, I.T.; Maser, W.K.; Benito, A.M.; Coleman, J.N. Improving the mechanical properties of graphene oxide based materials by covalent attachment of polymer chains. Carbon 2013, 52, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yan, H.; Yang, X.; Liu, C. Effect of functionalized graphene oxide with a hyperbranched cyclotriphosphazene polymer on mechanical and thermal properties of cyanate ester composites. RSC Adv. 2014, 4, 45930–45938. [Google Scholar] [CrossRef]
- Forati, T.; Atai, M.; Rashidi, A.M.; Imani, M.; Behnamghader, A. Physical and mechanical properties of graphene oxide/polyethersulfone nanocomposites. Polym. Adv. Technol. 2014, 25, 322–328. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liang, S.; Yu, L.Y.; Skov, A.L.; Etmimi, H.M.; Mallon, P.E.; Adronov, A.; Brook, M.A. Silicone-modified graphene oxide fillers via the piers-rubinsztajn reaction. J. Polym. Sci. Pol. Chem. 2016, 54, 2379–2385. [Google Scholar] [CrossRef]









| Polymers | T5%/°C | Tg/°C | ΔHrelaxation/J·g−1 |
|---|---|---|---|
| PDMS-co-MHS-1 | 140 | - | - |
| 0.75:1 a PDP:PDMS-co-MHS-1 | 177 | 45-50 | 28.5 |
| 0.75:1 a TOP:PDMS-co-MHS-1 | 263 | - | - |
| 0.75:1 a phenol:PDMS-co-MHS-1 | 152 | - | - |
| PDMS-co-MHS-2 | 150 | - | - |
| 0.75:1 a PDP:PDMS-co-MHS-2 | 244 | - | - |
| 0.75:1 a TOP:PDMS-co-MHS-2 | 130 | 75-80 | 2.8 |
| 0.75:1 a phenol:PDMS-co-MHS-2 | 150 | - | - |
| Lignin-Silicone Elastomer (LE-#) | Shore OO | Shore A | Modulus/MPa | Elongation at Break/% |
|---|---|---|---|---|
| LE-0.5 | 35–45 | - | - | - |
| LE-27 | 85–90 | 33–35 | 0.93 ± 0.22 | 286 ± 40 |
| LE-41A | 85–90 | 35–40 | 1.31 ± 0.02 | 260 ± 14 |
| LE-41B | 85–90 | 45–50 | 3.28 ± 0.39 | 146 ± 17 |
| LE-41F | 85–90 | 45–50 | 1.95 ± 0.49 | 297 ± 30 |
| LE-41G | 70–75 | 20–25 | 0.15 ± 0.01 | 305 ± 21 |
| LE-66 | 85–90 | 75–85 | - | - |
| Samples | Dielectric Constant (Dk) | Thermostability | Water Uptake (%) | Storage Modulus (GPa) |
|---|---|---|---|---|
| Cured BCB-Si-E | 2.77 | T5% of 400 °C | 0.15 | 5.9 |
| Commercial bisphenol A-type epoxy resin | - a | - a | 1.25 | - a |
| Anethole-based BCB resin | 2.9 | - a | - a | - a |
| Polybenzoxazine resins | 2.81 | - a | - a | - a |
| BCB resins | 2.8 | - a | - a | - a |
| Softwood-lignin-based polycarbonates | - a | T5% of 346 °C | - a | - a |
| Softwood-lignin-based cyanate esters | - a | T5% of 375 °C | - a | - a |
| Sample | Percentage of GO-PDMS (wt %) | Break Strength (MPa) | Elongation at Break (%) | Oxygen Permeability Coefficient (mm·L·m−2 Day·Bar) |
|---|---|---|---|---|
| Control | 0 | 0.38 ± 0.09 | 103 ± 9 | 250 ± 0.6 % |
| 1 | 1 | 0.27 ± 0.04 | 79 ± 11 | 273 ± 0.4 % |
| 2 | 3 | 0.19 ± 0.02 | 144 ± 29 | 220 ± 0.4 % |
| 3 | 5 | 0.51 ± 0.04 | 247 ± 38 | 193 ± 0.5 % |
| 4 | 10 | 0.48 ± 0.22 | 202 ± 76 | 135 ± 0.6 % |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yi, M.; Wu, S.; Tan, L.; Ge, X.; He, M.; Yin, G. Synthesis of Structurally Precise Polysiloxanes via the Piers–Rubinsztajn Reaction. Materials 2019, 12, 304. https://doi.org/10.3390/ma12020304
Chen X, Yi M, Wu S, Tan L, Ge X, He M, Yin G. Synthesis of Structurally Precise Polysiloxanes via the Piers–Rubinsztajn Reaction. Materials. 2019; 12(2):304. https://doi.org/10.3390/ma12020304
Chicago/Turabian StyleChen, Xunjun, Minghao Yi, Shufang Wu, Lewen Tan, Xin Ge, Ming He, and Guoqiang Yin. 2019. "Synthesis of Structurally Precise Polysiloxanes via the Piers–Rubinsztajn Reaction" Materials 12, no. 2: 304. https://doi.org/10.3390/ma12020304
APA StyleChen, X., Yi, M., Wu, S., Tan, L., Ge, X., He, M., & Yin, G. (2019). Synthesis of Structurally Precise Polysiloxanes via the Piers–Rubinsztajn Reaction. Materials, 12(2), 304. https://doi.org/10.3390/ma12020304