Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Layered Li2TiO3and H2TiO3
2.2. Exfoliation of H2TiO3 into a Nanosheet Colloidal Solution
2.3. Microwave Hydrothermal Synthesis of TiO2 Nanocrystals
2.4. Photocatalytic Activity
2.5. Fabrication of Photoanodes and Dye-Sensitized Solar Cells (DSSCs)
2.6. Characterization
3. Results and Discussion
3.1. Preparation of the Metatitanic Acid Titanate Nanosheet Colloidal Solution
3.2. Synthesis of TiO2 Nanocrystals from Metatitanic Acid Nanosheet Colloidal Solution
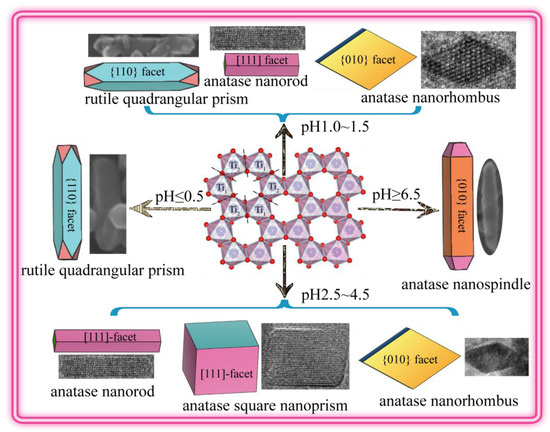
3.3. Morphology and Exposed Crystal Facets of TiO2 Nanocrystals
3.4. Transformation Reaction from the Delaminated H2TiO3 Nanosheets to TO2 Nanocrystals
3.5. Photocatalytic Activities of the As-Synthesized TiO2 Nanocrystals
3.6. Photovoltic Performances of As-Synthesized TiO2 Nanocrystals
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Yang, B.X.; Yang, X.H.; Chen, Z.G.; Li, Z.; Zhao, H.J.; Gong, X.G.; Yang, H.G. On the synergistic effect of hydrohalic acids in the shape-controlled synthesis of anatase TiO2 single crystals. Cryst. Eng. Comm. 2013, 15, 3252–3255. [Google Scholar] [CrossRef]
- Wen, C.Z.; Jiang, H.B.; Qiao, S.Z.; Yang, H.G.; Lu, G.Q. Synthesis of high-reactive facets dominated anatase TiO2. J. Mater. Chem. 2011, 21, 7052–7061. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.L.; Shi, Y.X.; Xu, G.L.; Zhang, E.P.; Wang, H.B.; Kong, Z.; Xia, J.H.; Ji, Z.G. Anatase TiO2 nanosheets with coexposed {101} and {001}facets coupled with ultrathin SnS2 nanosheets as a face-to-face n-p-n dual heterojunction photocatalyst for enhancing photocatalytic activity. Appl. Surf. Sci. 2017, 420, 839–848. [Google Scholar] [CrossRef]
- Regue, M.; Sibby, S.; Ahmet, I.Y.; Friedrich, D.; Abdi, F.F.; Johnson, A.L.; Eslava, S. TiO2 photoanodes with exposed {010} facets grown by aerosol-assisted chemical vapor deposition of a titanium oxo/alkoxy cluster. J. Mater. Chem. A 2019, 7, 19161–19172. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Olivier, C.; Majimel, J.; Klein, A.; Jaegermann, W.; Toupance, T. Nickel oxide selectively depositedon the {101} facet of anatase TiO2 nanocrystal bipyramids for enhanced photocatalysis. ACS Appl. Nano Mater. 2019, 2, 4793–4803. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-E.; Bai, Y.; An, J.; Cai, X.M.; Chen, Y.Q.; Wang, P.F.; Yang, X.J.; Feng, Q. Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, T.T.; Liu, C.; Yang, Y.; Pan, J.H.; Yao, J.X.; Hu, L.H.; Dai, S.Y. Shape-controlled synthesis of single-crystalline anatase TiO2 micro/nanoarchitectures for efficient dye-sensitized solar cells. Sustain. Energy Fuels 2017, 1, 520–528. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T.; Mohamed, A.R. Highly reactive {001} facets of TiO2-based composites: synthesis, formation mechanism and characterization. Nanoscale 2014, 6, 1946–2008. [Google Scholar] [CrossRef]
- Du, Y.-E.; Niu, X.J.; Bai, Y.; Qi, H.X.; Guo, Y.Q.; Chen, Y.Q.; Wang, P.F.; Yang, X.J.; Feng, Q. Synthesis of anatase TiO2 nanocrystals with defined morphologies from exfoliated nanoribbons: photocatalytic performance and application in dye-sensitized solar cell. ChemistrySelect 2019, 4, 4443–4457. [Google Scholar] [CrossRef]
- Yu, S.L.; Liu, B.C.; Wang, Q.; Gao, Y.X.; Shi, Y.; Feng, X.; An, X.T.; Liu, L.X.; Zhang, J. Ionic liquid assisted chemical strategy to TiO2 hollow nanocube assemblies with surface-fluorination and nitridation and high energy crystal facet exposure for enhanced photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 10283–10295. [Google Scholar] [CrossRef]
- Yu, J.G.; Wang, G.H.; Cheng, B.; Zhou, M.H. Effects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders. Appl. Catal. B 2007, 69, 171–180. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.M.; Han, Y.H.; Liu, P.R.; Yao, X.D.; Zhao, H.J. A selective etching phenomenon on {001} faceted anatase titanium dioxide single crystal surfaces by hydrofluoric acid. Chem. Commun. 2011, 47, 2829–2831. [Google Scholar] [CrossRef] [PubMed]
- Han, X.G.; Kuang, Q.; Jin, M.S.; Xie, Z.X.; Zheng, L.S. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Reunchan, P.; Ouyang, S.X.; Tong, H.; Umezawa, N.; Kako, T.; Ye, J.H. Anatase TiO2 single crystals exposed with high-reactive {111} facets toward efficient H2 evolution. Chem. Mater. 2013, 25, 405–411. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Wen, P.H.; Itoh, H.; Tang, W.P.; Feng, Q. Single nanocrystals of anatase-type TiO2 prepared from layered titanate nanosheets: Formation mechanism and characterization of surface properties. Langmuir 2007, 23, 11782–11790. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal Synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef]
- Ye, L.Q.; Mao, J.; Liu, J.Y.; Jiang, Z.; Peng, T.Y.; Zan, L. Synthesis of anatase TiO2 nanocrystals with {101}, {001} or {010} single facets of 90% level exposure and liquidphase photocatalytic reduction and oxidation activity orders. J. Mater. Chem. A 2013, 1, 10532–10537. [Google Scholar] [CrossRef]
- Li, T.; Shen, Z.L.; Shu, Y.L.; Li, X.G.; Jiang, C.J.; Chen, W. Facet-dependent evolution of surface defects in anatase TiO2 by thermal treatment: Implications for environmental applications of photocatalysis. Environ. Sci. Nano 2019, 6, 1740–1753. [Google Scholar] [CrossRef]
- Feng, J.Y.; Yin, M.C.; Wang, Z.Q.; Yan, S.C.; Wan, L.J.; Li, Z.S.; Zou, Z.G. Facile synthesis of anatase TiO2 mesocrystal sheets with dominant {001} facets based on topochemical conversion. CrystEngComm 2010, 12, 3425–3429. [Google Scholar] [CrossRef]
- Illa, S.; Boppella, R.; Manorama, S.V.; Basak, P. Mesoporous assembly of cuboid anatase nanocrystals into hollow spheres: Realizing enhanced photoactivity of high energy {001} facets. J. Phys. Chem. C 2016, 120, 18028–18038. [Google Scholar] [CrossRef]
- Khalil, M.; Anggraeni, E.S.; Ivandini, T.A.; Budianto, E. Exposing TiO2 (001) crystal facet in nano Au-TiO2 heterostructures for enhanced photodegradation of methylene blue. Appl. Surf. Sci. 2019, 487, 1376–1384. [Google Scholar] [CrossRef]
- Ye, L.Q.; Liu, J.Y.; Tian, L.H.; Peng, T.Y.; Zan, L. The replacement of {101} by {010} facets inhibits the photocatalytic activity of anatase TiO2. Appl. Catal., B 2013, 134–135, 7515–7519. [Google Scholar] [CrossRef]
- Amoli, V.; Bhat, S.; Maurya, A.; Banerjee, B.; Bhaumik, A.; Sinha, A.K. Tailored synthesis of porous TiO2nanocubes and nanoparallelepipeds with exposed {111} facets and mesoscopic void space: A superior candidate for efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 26022–26035. [Google Scholar] [CrossRef]
- Niu, X.J.; Du, Y.E.; Liu, Y.F.; Qi, H.X.; An, J.; Yang, X.J.; Feng, Q. Hydrothermal synthesis and formation mechanism of the anatase nanocrystals with co-exposed highenergy {001}, {010} and [111]-facets for enhanced photocatalytic performance. RSC Adv. 2017, 7, 24616–24627. [Google Scholar] [CrossRef]
- Liu, Y.F.; Du, Y.-E.; Bai, Y.; An, J.; Li, J.Q.; Yang, X.J.; Feng, Q. Facile synthesis of {101}, {010} and [111]-Faceted anatase-TiO2 nanocrystals derived from porous metatitanic acid H2TiO3 for enhanced photocatalytic performance. ChemistrySelect 2018, 3, 2867–2876. [Google Scholar] [CrossRef]
- Gerbec, J.A.; Magana, D.; Washington, A.; Strouse, G.F. Microwave-enhanced reaction rates for nanoparticle synthesis. J. Am. Chem. Soc. 2005, 127, 15791–15800. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, E.-S.; Manthiram, A. Microwave-solvothermal synthesis of various polymorphs of nanostructured TiO2 in different alcohol media and their lithium ion storage properties. Inorg. Chem. 2012, 51, 3505–3512. [Google Scholar] [CrossRef]
- Krishnapriya, R.; Praneetha, S.; Murugan, A.V. Microwave-solvothermal synthesis of different TiO2 nanomorphologies with perked up efficiency escorted by incorporating Ni nanoparticle in electrolyte for dye sensitized solar cells. Inorg. Chem. Front. 2017, 4, 1665–1678. [Google Scholar] [CrossRef]
- Kokel, A.; Schäfer, C.; Török, B. Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem. 2017, 19, 3729–3751. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Santos, L.; Barquinha, P.; Fortunato, E.; Rodrigo Martins, R. Photocatalytic TiO2 nanorod spheres and arrays compatible with flexible applications. Catalysts 2017, 7, 60. [Google Scholar] [CrossRef]
- Shi, X.C.; Zhang, Z.B.; Zhou, D.F.; Zhang, L.F.; Chen, B.Z.; Yu, L.L. Synthesis of Li+ adsorbent (H2TiO3) and its adsorption properties. Trans. Nonferrous Met. Soc. China 2013, 23, 253–259. [Google Scholar] [CrossRef]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G.S. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Liao, Y.L.; Que, W.X.; Jia, Q.Y.; He, Y.C.; Zhang, J.; Zhong, P. Controllable synthesis of brookite/anatase/rutile TiO2 nanocomposites and single-crystalline rutile nanorods array. J. Mater. Chem. 2012, 22, 7937–7944. [Google Scholar] [CrossRef]
- Zheng, J.-Y.; Bao, S.-H.; Guo, Y.; Jin, P. Anatase TiO2 films with dominant {001} facets fabricated by direct-current reactive magnetron sputtering at room temperature: oxygen defects and enhanced visible-light photocatalytic behaviors. ACS Appl. Mater. Interfaces 2014, 6, 5940–5946. [Google Scholar] [CrossRef]
- Azuma, K.; Dover, C.; Grinter, D.C.; Grau-Grespo, R.; Almora-Barrios, N.; Thornton, G.; Oda, T.; Tanaka, S. Scanning tunneling microscopy and molecular dynamics study of the Li2TiO3 (001) surface. J. Phys. Chem. C 2013, 117, 5126–5131. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Lithium recovery from salt brine H2TiO3. Dalton Trans. 2014, 43, 8933–8939. [Google Scholar] [CrossRef]
- Yu, C.-L.; Yanagisawa, K.; Kamiya, S.; Kozawa, T.; Ueda, T. Monoclinic Li2TiO3nano-particles via hydrothermal reaction: processing and structure. Ceram. Int. 2014, 40, 1901–1908. [Google Scholar] [CrossRef]
- Hou, W.H.; Chen, Y.S.; Guo, C.X.; Yan, Q.J. Synthesis of porous chromia-pillared tetratitanate. J. Solid State Chem. 1998, 136, 320–321. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Ooi, K.; Kanoh, H.; Tang, W.-P.; Tomida, T. Swelling and delamination behaviors of birnessite-type manganese oxide by intercalation of tetraalkylammonium ions. Langmuir 2000, 16, 4154–4164. [Google Scholar] [CrossRef]
- Allen, M.R.; Thibert, A.; Sabio, E.M.; Browning, N.D.; Larsen, D.S.; Osterloh, F.E. Evolution of physical and photocatalytic properties in the layered titanates A2Ti4O9 (A = K, H) and nanosheets derived by chemical exfoliation. Chem. Mater. 2010, 22, 1220–1228. [Google Scholar] [CrossRef]
- Pal, S.; Laera, A.M.; Licciulli, A.; Catalano, M.; Taurino, A. Biphase TiO2 microspheres with enhanced photocatalytic activity. Ind. Eng. Chem. Res. 2014, 53, 7931–7938. [Google Scholar] [CrossRef]
- Liu, S.W.; Yu, J.G.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedral with exposed {001} facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef]
- Yang, W.G.; Wang, Y.L.; Shi, W.M. One-step synthesis of single-crystal anatase TiO2 tetragonal faceted-nanorods for improved-performance dye-sensitized solar cells. CrystEngComm 2012, 14, 230–234. [Google Scholar] [CrossRef]
- Zuo, F.; Dillon, R.J.; Wang, L.; Smith, P.; Zhao, X.; Bardeen, C.; Feng, P. Active facets on titanium(III)-doped TiO2: A effective strategy to improve the visible-light photocatalytic. Angew. Chem., Int. Ed. 2012, 51, 6223–6226. [Google Scholar] [CrossRef]
- Liu, B.; Khare, A.; Aydil, E.S. TiO2-B/anatase core-shell heterojunction nanowires for photocatalysis. ACS Appl. Mater. Inter. 2011, 3, 4444–4450. [Google Scholar] [CrossRef]
- Wu, T.X.; Liu, G.M.; Zhao, J.C. Photoassisted degradation of dye pollutants. v. self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation. J. Photochem. Photobiol. A Chem. 2001, 140, 163–172. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Deng, D.; Chen, N.; Xing, X.; Wang, Y. A one-step nonaqueous sol–gel route to mixed-phase TiO2 with enhanced photocatalytic degradation of rhodamine B under visible light. CrystEngComm. 2016, 18, 1964–1975. [Google Scholar] [CrossRef]
- Zhang, H.M.; Liu, P.R.; Li, F.; Liu, H.W.; Wang, Y.; Zhang, S.Q.; Guo, M.X.; Cheng, H.M.; Zhao, H.J. Facile fabrication of anatase TiO2 microspheres on solid substrates and surface crystal facet transformation from {001} and {100}. Chem. Eur. J. 2011, 17, 5949–5957. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.W.; Li, G.D.; Wang, Y.; Zeng, S.J.; Yan, Z.J.; Wang, J.W.; Xin, S.G.; Zhang, L.; Wu, S.W.; Zhang, Z.T. Facile formation of mesoporous structured mixed-phase (anatase/rutile) TiO2 with enhanced visible light photocatalytic activity. Chem. Commun. 2018, 54, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.W.; Zhang, W.X.; Tanaka, Y.; Kusunose, N.; Peng, Y.; Feeng, Q. Mesocrystalline nanocomposition of TiO2 polymorphs: Topochemical mesocrystal conversion, characterization, and photocatalytic response. Cryst. Growth Des. 2015, 15, 1214–1225. [Google Scholar] [CrossRef]
- Du, Y.-E.; Bai, Y.; Liu, Y.F.; Guo, Y.Q.; Cai, X.M.; Feng, Q. One-pot synthesis of [111]-/{010} facets coexisting anatase nanocrystals with enhanced dye-sensitized solar cell performance. ChemistrySelect 2016, 1, 6632–6640. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.Y.; Zhao, L.; Ju, S.T.; Yan, Z.J.; He, T.; Zhou, C.L.; Wang, W.J. Anatase TiO2 single crystals with exposed {001} and {110} facets: facile synthesis and enhanced photocatalysis. Chem. Commun. 2010, 46, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.H.; Xue, M.; Ishikawa, Y.; Itoh, H.; Feng, Q. Relationships between cell parameters of dye-sensitized solar cells and dye-adsorption parameters. ACS Appl. Mater. Interfaces 2012, 4, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Zheng, L.X.; Liu, C.P.; Liu, Y.K.; Luan, C.Y.; Cheng, H.; Li, Y.Y.; Martinu, L.; Zapien, J.A.; Bello, I. Rapid microwave synthesis of porous TiO2 spheres and their applications in dye-sensitized solar cells. J. Phys. Chem. C 2011, 115, 10419–10425. [Google Scholar] [CrossRef]
- Chen, J.Z.; Li, B.; Zheng, J.F.; Jia, S.P.; Zhao, J.H.; Jing, H.W.; Zhu, Z.P. Role of one-dimensional ribbonlike nanostructures in dye-sensitized TiO2-based solar cells. J. Phys. Chem. C 2011, 115, 7104–7113. [Google Scholar] [CrossRef]
- Chen, C.D.; Sewvandi, G.A.; Kusunose, T.; Tanaka, Y.; Nakanishi, S.; Feng, Q. Synthesis of {010}-faceted anatase TiO2 nanoparticles from layered titanate for dye-sensitized solar cells. CrystEngComm 2014, 16, 8885–8895. [Google Scholar] [CrossRef]
- Du, Y.-E.; Feng, Q.; Chen, C.D.; Tanaka, Y.; Yang, X.J. Photocatalytic and dye-sensitized solar cell performances of {010}-faceted and [111]-faceted anatase TiO2 nanocrystals synthesized from tetratitanate nanoribbons. ACS Appl. Mater. Interfaces 2014, 6, 16007–16019. [Google Scholar] [CrossRef]













| Samples | Specific Surface Area (m2/g) | Average Particle Size (nm) | Degradation Rate Constant k (min−1) | Correlative Coefficient (R2) |
|---|---|---|---|---|
| pH2.5-T175 | 135.6 | 8.3 | 0.0346 | 0.9502 |
| pH4.5-T175 | 54.8 | 13.3 | 0.0281 | 0.9846 |
| pH6.5-T175 | 32.3 | 87.0 | 0.0065 | 0.9920 |
| P25 | 52.5 | 23.9 | 0.0187 | 0.9971 |
| blank | 0.0004 | 0.9595 |
| Photoelectrode | Film Thickness (μm) | JSC (mA/cm2) | VOC (V) | FF | η (%) |
|---|---|---|---|---|---|
| pH2.5-T175 | 19.17 | 13.19 | 0.701 | 0.595 | 5.50 |
| pH4.5-T175 | 20.03 | 12.32 | 0.704 | 0.556 | 4.83 |
| pH6.5-T175 | 19.11 | 8.22 | 0.715 | 0.622 | 3.66 |
| P25 | 20.40 | 10.73 | 0.693 | 0.554 | 4.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.-e.; Niu, X.; Li, W.; An, J.; Liu, Y.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance. Materials 2019, 12, 3614. https://doi.org/10.3390/ma12213614
Du Y-e, Niu X, Li W, An J, Liu Y, Chen Y, Wang P, Yang X, Feng Q. Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance. Materials. 2019; 12(21):3614. https://doi.org/10.3390/ma12213614
Chicago/Turabian StyleDu, Yi-en, Xianjun Niu, Wanxi Li, Jing An, Yufang Liu, Yongqiang Chen, Pengfei Wang, Xiaojing Yang, and Qi Feng. 2019. "Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance" Materials 12, no. 21: 3614. https://doi.org/10.3390/ma12213614
APA StyleDu, Y. -e., Niu, X., Li, W., An, J., Liu, Y., Chen, Y., Wang, P., Yang, X., & Feng, Q. (2019). Microwave-Assisted Synthesis of High-Energy Faceted TiO2 Nanocrystals Derived from Exfoliated Porous Metatitanic Acid Nanosheets with Improved Photocatalytic and Photovoltaic Performance. Materials, 12(21), 3614. https://doi.org/10.3390/ma12213614