Efficiency of Novel Photocatalytic Coating and Consolidants for Protection of Valuable Mineral Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Substrates
2.2. Materials and Application on the Substrates
2.2.1. Consolidants
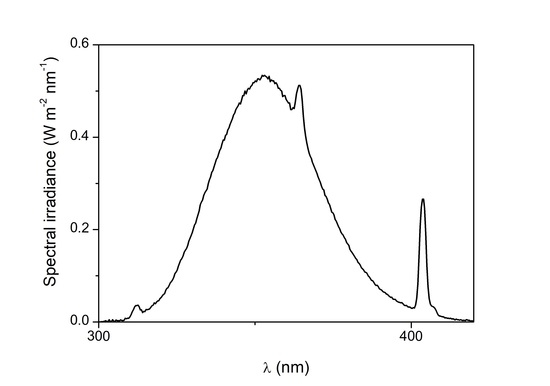
2.2.2. Photocatalytic Coating
2.3. Methods
3. Results and Discussion
3.1. Consolidants
3.2. Photocatalytic Coating
4. Conclusions
- ●
- In the case of silicate-based consolidant, generally a low risk of chromatic incompatibility (∆E* < 3) between the nontreated and treated substrates was shown. We found a decrease in the volume of removed material (increased abrasion resistance) after treatment, as well as an increase in the DRMS resistance through the profile of the substrates, indicating greater hardness of the treated substrates. After consolidation, the substrates showed a decrease of porosity of the consolidated substrate, while water vapour permeability did not change;
- ●
- Treatment with a carbonate-based consolidant showed no whitening of the surface, with low risk of chromatic incompatibility (∆E* < 3) between the nontreated and the treated mineral substrates. The study showed enhancement of consolidation after treatment, by a decrease in the volume of removed material after consolidation and by a small increase in DRMS resistance. Carbonate-based consolidation had a negligible effect on the pore structure of the mineral substrates, as well as on their water vapour permeability;
- ●
- The newly designed photocatalytic suspension based on TiO2/LDH showed negligible changes in the water vapour permeability and colour change values compared to the nontreated substrates. Besides good compatibility, the obtained results indicate good durability of the developed protective TiO2/LDH coatings, as well as a strong impact on the photocatalytic properties of the porous building materials, even after the durability tests involving rinsing and freezing/thawing procedures.
Author Contributions
Funding
Conflicts of Interest
References
- Baglioni, P.; Giorgi, R. Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter 2006, 2, 293–303. [Google Scholar] [CrossRef]
- Salvadori, B.; Dei, L. Synthesis of Ca(OH)2 nanoparticles from diols. Langmuir 2001, 17, 2371–2374. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G.; Quaresima, R. The nanolimes in cultural heritage conservation: Characterization and analysis of the carbonation process. J. Cult. Herit. 2008, 9, 294–301. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage. A Compedium of Materials and Techniques, 1st ed.; Springer: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ambrosi, M.; Dei, L.; Giorgi, R.; Neto, C.; Baglioni, P. Colloidal particles of Ca(OH2). Properties and applications to restoration of frescoes. Langmuir 2001, 17, 4251–4255. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Carreti, E.; Toccafondi, N.; Jaidar, Y. Commercial Ca(OH)2 nanoparticles for the consolidation of immovable works of art. Appl. Phys. A 2014, 114, 723–732. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Suzuki, A.; Ruiz-Agudo, E. Alcohol dispersions of calcium hydroxide nanoparticles for stone conservation. Langmuir 2013, 29, 11457–11470. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, S.; La Russa, M.F.; Aloise, P.; Belfiore, C.M.; Macchia, A.; Pezzino, A.; Crisci, G.M. Efficacy of nanolime in restoration procedures of salt weathered limestone rocks. Appl. Phys. A 2014, 114, 753–758. [Google Scholar] [CrossRef]
- Pondelak, A.; Kramar, S.; Lesar Kikelj, M.; Sever Škapin, A. In-situ study of the consolidation of wall paintings using commercial and newly developed consolidants. J. Cult. Herit. 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Škrlep, L.; Pondelak, A.; Sever Škapin, A. Method for Reinforcing Porous Construction Materials and Use Calcium Acetoacetate Solution to this Aim: International Application No. PCT/SI2014/000028. [S. l.]: EU Pat. 3004028 B1, 5. 4. 2017. Available online: https://register.epo.org/application?number=EP14738917&lng=en&tab=family (accessed on 9 February 2019).
- Ropret, P.; Legan, L.; Retko, K.; Špec, T.; Pondelak, A.; Škrlep, L.; Sever-Škapin, A. Evaluation of vibrational spectroscopic techniques for consolidants´ penetration depth determination. J. Cult. Herit. 2017, 23, 148–156. [Google Scholar] [CrossRef]
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone; The Getty Conservation Institute: Los Angeles, CA, USA, 2005. [Google Scholar]
- Sherer, G.W.; Wheeler, G.S. Silicate consolidant for stone. Key Eng. Mater. 2009, 391, 1–25. [Google Scholar] [CrossRef]
- Franzoni, E.; Graziani, G.; Sassoni, E.; Bacilieri, G.; Griffa, M.; Lura, P. Solvent-based ethyl silicate for stone consolidation; Influence of the application technique on penetration depth, efficacy and pore occlusion. Mater. Struct. 2015, 48, 3503–3515. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Franzoni, E. Consolidation of porous carbonate stones by innovative phosphate treatment: Mechanical strengthening and physical-microstructural compatibility in comparison with TEOS-based treatments. Herit. Sci. 2015, 3, 1. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Kallithrakas-Kontos, N.; Agioutantis, Z.; Maurigiannakis, S.; Korakaki, D. A comparative study of porous limestones treated with silicon-based strengthening agents. Prog. Org. Coat. 2008, 62, 49–60. [Google Scholar] [CrossRef]
- Miliani, C.; Velo-Simpson, M.L.; Sherer, G.W. Particle modified consolidants: A study on the effect of particles on sol-gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Zendri, E.; Biscontin, G.; Nardini, I.; Riato, S. Characterization and reactivity of silicatic consolidants. Constr. Build. Mater. 2007, 21, 1098–1106. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.K.; Won, J.; Do, J.Y.; Kim, S.D.; Kang, Y.S. Effects of silica nanoparticle and GPTMS addition on TEOS-based stone consolidants. J. Cult. Herit. 2009, 10, 214–221. [Google Scholar] [CrossRef]
- Ksinopolou, E.; Bakolas, A.; Moropulou, A. Modifying Si-based consolidants through the addition of colloidal nano-particles. Appl. Phys. A 2016, 122, 267. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Ormellese, M.; Pedeferri, M.P. Photocatalytic and self-cleaning activity of colored mortars containing TiO2. Constr. Build. Mater. 2013, 46, 167–174. [Google Scholar] [CrossRef]
- Quagliarini, F.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafo, P. Smart surfaces for architectural heritage: Preliminary results about the application of TiO2-based coating on travertine. J. Cult. Herit. 2012, 13, 204–209. [Google Scholar] [CrossRef]
- Enea, D. Recent Development on Self-Cleaning Cementitious Coatings, in Self-Cleaning Materials and Surfaces: A Nanotechnology Approach; Daoud, W.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar] [CrossRef]
- Warheit, D.B. Nanoparticles: Health impact? Mater. Today 2004, 7, 32–35. [Google Scholar] [CrossRef]
- Bianchi, C.; Gatto, S.; Pirola, C.; Scavini, M.; Vitali, S.; Capucci, V. Micro-TiO2 as a starting material for new photocatalytic tiles. Cem. Concr. Compos. 2013, 36, 116–120. [Google Scholar] [CrossRef]
- Rudic, O.; Rajnovic, D.; Cjepa, D.; Vucetic, S.; Ranogajec, J. Investigation of the durability of porous mineral substrates with newly designed TiO2-LDH coating. Ceram. Int. 2015, 41, 9779–9792. [Google Scholar] [CrossRef]
- Todorova, N.; Giannakopoulou, T.; Karapati, S.; Petridis, D.; Vaimakis, T.; Trapalis, C. Composite TiO2/clays materials for photocatalytic NOx oxidation. Appl. Surf. Sci. 2014, 319, 113–120. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Manilla, A.; Banuelos, F.; Fernandez, J.L.; Gomez, R. Improved photocatalytic degradation of phenolic compounds with Zn Al mixed oxides obtained from LDH materials. Top. Catal. 2011, 54, 257–263. [Google Scholar] [CrossRef]
- “Protection of Cultural Heritage Objects with Multifunctional Advanced Materials”—Acronym HEROMAT, granted by the European Commision in the Seventh Framework Programme (2007—2013), Grand agreement No. 282992. Available online: http://www.heromat.com/ (accessed on 9 August 2016).
- Kramar, S.; Ducman, V.; Vucetic, S.; Velkavrh, E.; Radeka, M.; Ranogajec, J. Characterization of the substrates from two cultural-heritage sites and a preparation of model substrates. Mater. Technol. 2014, 48, 505–508. [Google Scholar]
- Franzoni, E.; Graziani, G.; Sassoni, E. TEOS-based treatments for stone consolidation: Acceleration of hydrolysis–condensation reactions by poulticing. J. Sol-Gel Sci. Technol. 2015, 74, 398–405. [Google Scholar] [CrossRef]
- Ranogajec, J.; Rudic, O.; Vucetic, S. Nanocomposite photocatalyst based on layered double hydroxides (LDHs) associated with TiO2. In Proceedings of the 13th International Conference on Modern Materials and Technologies, Montecatini Terme, Italy, 8–13 June 2014. [Google Scholar]
- Mokrzycki, W.; Tatol, M. Colour Difference ∆E—A Survey; University of Warmia and Mazury: Olsztyn, Poland, 2011; pp. 383–411. [Google Scholar]
- HunterLab Colour Theory: Colour Scales. Available online: https://support.hunterlab.com/ (accessed on 5 July 2016).
- EN ISO 10545-6/2012: Ceramic Tiles. Determination of Resistance to Deep Abrasion for Unglazed Tiles; BSI: London, UK, 2012.
- EN ISO 12572:2002: Hygrothermal Performance of Building Materials and Products—Determination of Water Vapour Transmission Properties; BSI: London, UK, 2002.
- Tasbihi, M.; Lavrenčić Štangar, U.; Sever-Škapin, A.; Ristić, A.; Kaučič, V.; Novak, T.N. Titania-containing mesoporous silica powders: Structural properties and photocatalytic activity towards isopropanol degradation. J. Photochem. Photobiol. A 2010, 216, 167–178. [Google Scholar] [CrossRef]
- Vučetić, S.B.; Rudić, O.L.; Markov, S.L.; Bera, O.J.; Vidaković, A.M.; Skapin, A.S.; Ranogajec, J.G. Antifungal efficiency assessment of the TiO2 coating on façade paints. Environ. Sci. Pollut. Res. 2014, 21, 11228–11237. [Google Scholar] [CrossRef]
- Vidaković, A.M.; Ranogajec, J.G.; Markov, M.S.L.; Lončar, E.S.; Hirsenberger, H.M.; Sever-Škapin, A. Synergistic effect of the consolidant and the photocatalytic coating on antifungal activity of porous mineral substrates. J. Cult. Herit. 2017, 124, 1–8. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- BS EN 12371:2010 Natural Stone Test Methods. Determination of Frost Resistance; BSI: London, UK, 2010.
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Kopelson, E. Analysis and Consolidation of Architectural Plaster from Catalhoyuk, Turkey. Master’s Thesis, University of Pennsylvania, Philadelphia, PA, USA, 1996. [Google Scholar]
- Young, M.E.; Murray, M.; Cordiner, P. Stone Consolidants and Chemical Treatments in Scotland; Historic Scotland: Edinburg, TX, USA, 1999. [Google Scholar]







| Substrate Treated with Consolidant or Coating | ∆L* | ∆a* | ∆b* | ∆E* |
|---|---|---|---|---|
| brick CF4 | −3.63 | +0.55 | +0.65 | 3.7 |
| stone CF4 | −2.9 | +0.13 | +0.23 | 2.9 |
| mortar CFW | −1.27 | −0.34 | +2.63 | 2.9 |
| render CFW | −1.16 | +0.56 | +2.33 | 2.7 |
| brick PF | −0.09 | −0.01 | −0.80 | 1.0 |
| stone PF | +0.21 | −0.15 | +0.23 | 0.8 |
| mortar PF | −0.36 | −0.30 | +0.49 | 1.1 |
| render PF | −0.21 | −0.15 | +0.23 | 0.6 |
| Substrate with or without Consolidant | Length L (mm) | Volume of the Removed Material V (mm3) |
|---|---|---|
| brick | 52.0 ± 2.0 | 1202 ± 140 |
| brick CF4 | 46.8 ± 1.3 | 868 ± 70 |
| stone | 34.8 ± 0.4 | 353 ± 8 |
| stone CF4 | 34.0 ± 0.5 | 335 ± 15 |
| mortar | 83.8 ± 3.8 | 5179 ± 698 |
| mortar CFW | 68.3 ± 0.3 | 2748 ± 31 |
| render | 25.3 ± 0.3 | 135 ± 12 |
| render CFW | 23.5 ± 0.5 | 109 ± 7 |
| Substrate with and without Consolidant | Brick | Brick CF4 | Stone | Stone CF4 | Mortar | Mortar CFW | Render | Render CFW | |
|---|---|---|---|---|---|---|---|---|---|
| Porosity (%) | 44.2 ± 0.2 | 40.2 ± 0.1 | 12.3 ± 0.2 | 9.8 ± 0.7 | 30.2 ± 0.5 | 30.8 ± 0.2 | 26.7 ± 0.2 | 26.0 ± 0.4 | |
| BET surface area (m2/g) | 1.26 ± 0.0 | 0.97 ± 0.1 | 1.99 ± 0.1 | 0.36 ± 0.0 | 2.21 ± 0.1 | 2.02 ± 0.2 | 6.13 ± 1.1 | 5.11 ± 0.1 |
| Substrate with and without the Consolidant or Coating | Water Vapour Permeability µ (/) |
|---|---|
| brick | 31.9 ± 1.6 |
| brick CF4 | 31.9 ± 2.4 |
| brick PF | 31.5 ± 1.5 |
| mortar | 23.8 ± 1.9 |
| mortar CFW | 23.9 ± 1.7 |
| mortar PF | 23.7 ± 2.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pondelak, A.; Kramar, S.; Ranogajec, J.; Škrlep, L.; Vucetic, S.; Ducman, V.; Škapin, A.S. Efficiency of Novel Photocatalytic Coating and Consolidants for Protection of Valuable Mineral Substrates. Materials 2019, 12, 521. https://doi.org/10.3390/ma12030521
Pondelak A, Kramar S, Ranogajec J, Škrlep L, Vucetic S, Ducman V, Škapin AS. Efficiency of Novel Photocatalytic Coating and Consolidants for Protection of Valuable Mineral Substrates. Materials. 2019; 12(3):521. https://doi.org/10.3390/ma12030521
Chicago/Turabian StylePondelak, Andreja, Sabina Kramar, Jonjaua Ranogajec, Luka Škrlep, Snežana Vucetic, Vilma Ducman, and Andrijana Sever Škapin. 2019. "Efficiency of Novel Photocatalytic Coating and Consolidants for Protection of Valuable Mineral Substrates" Materials 12, no. 3: 521. https://doi.org/10.3390/ma12030521
APA StylePondelak, A., Kramar, S., Ranogajec, J., Škrlep, L., Vucetic, S., Ducman, V., & Škapin, A. S. (2019). Efficiency of Novel Photocatalytic Coating and Consolidants for Protection of Valuable Mineral Substrates. Materials, 12(3), 521. https://doi.org/10.3390/ma12030521