Investigation of the Corrosion Behavior of Atomic Layer Deposited Al2O3/TiO2 Nanolaminate Thin Films on Copper in 0.1 M NaCl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Substrate Preparation
2.1.2. Thin Film Deposition and Characterization
2.2. Electrochemistry
2.2.1. Electrochemical Impedance Spectroscopy
2.2.2. DC Voltammetry
3. Results and Discussion
3.1. Spectroscopic Ellipsometry
3.2. DC Voltammetry
3.2.1. Deoxygenated Electrolyte
3.2.2. Oxygenated Electrolyte
3.3. Electrochemical Impedance Spectroscopy
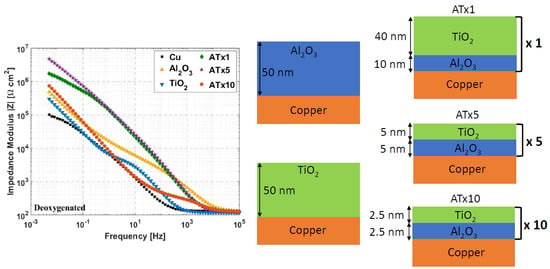
3.3.1. Deoxygenated Electrolyte
3.3.2. Oxygenated Electrolyte
3.4. ALD Film Stability
3.4.1. Deoxygenated Electrolyte
3.4.2. Oxygenated Electrolyte
3.5. Dissolution of Al2O3
3.6. Effect of Dissolved Oxygen
3.7. Copper-Based Surface Films and Sample Aging
3.8. Effect of Deposition Temperature
3.9. Nanolaminate Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Kharafi, F.M.; Shalaby, H.M. Corrosion Behavior of Annealed and Hard-Drawn Copper in Soft Tap Water. Corrosion 1995, 51, 469–481. [Google Scholar] [CrossRef]
- Sobue, K.; Sugahara, A.; Nakata, T.; Imai, H.; Magaino, S. Effect of free carbon dioxide on corrosion behavior of copper in simulated water. Surf. Coat. Technol. 2003, 169–170, 662–665. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, J.-G.; Koo, J.-Y. Investigation of pitting corrosion of a copper tube in a heating system. Eng. Fail. Anal. 2010, 17, 1424–1435. [Google Scholar] [CrossRef]
- Suh, S.H.; Suh, Y.; Yoon, H.G.; Oh, J.H.; Kim, Y.; Jung, K.; Kwon, H. Analysis of pitting corrosion failure of copper tubes in an apartment fire sprinkler system. Eng. Fail. Anal. 2016, 64, 111–125. [Google Scholar] [CrossRef]
- Lu, B.; Meng, W.J.; Mei, F. Experimental investigation of Cu-based, double-layered, microchannel heat exchangers. J. Micromech. Microeng. 2013, 23, 035017. [Google Scholar] [CrossRef]
- Mei, F.; Phillips, W.A.; Lu, B.; Meng, W.J.; Guo, S. Fabrication of copper-based microchannel devices and analysis of their flow and heat transfer characteristics. J. Micromech. Microeng. 2009, 19, 035009. [Google Scholar] [CrossRef]
- Medrano, M.; Yilmaz, M.O.; Nogués, M.; Martorell, I.; Roca, J.; Cabeza, L.F. Experimental evaluation of commercial heat exchangers for use as PCM thermal storage systems. Appl. Energy 2009, 86, 2047–2055. [Google Scholar] [CrossRef]
- Wang, X.J.; Qiu, X.; Ben-Zvi, I. Experimental observation of high-brightness microbunching in a photocathode rf electron gun. Phys. Rev. E 1996, 54, R3121–R3124. [Google Scholar] [CrossRef]
- Tooker, J.F.; Huynh, P.; Felch, K.; Blank, M.; Borchardt, P.; Cauffman, S. Gyrotron and power supply development for upgrading the electron cyclotron heating system on DIII-D. Fusion Eng. Des. 2013, 88, 521–524. [Google Scholar] [CrossRef]
- Musumeci, P.; Cultrera, L.; Ferrario, M.; Filippetto, D.; Gatti, G.; Gutierrez, M.S.; Moody, J.T.; Moore, N.; Rosenzweig, J.B.; Scoby, C.M.; et al. Multiphoton Photoemission from a Copper Cathode Illuminated by Ultrashort Laser Pulses in an rf Photoinjector. Phys. Rev. Lett. 2010, 104, 084801. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; To, H.; Andonian, G.; Feng, J.; Polyakov, A.; Scoby, C.M.; Thompson, K.; Wan, W.; Padmore, H.A.; Musumeci, P. Surface-Plasmon Resonance-Enhanced Multiphoton Emission of High-Brightness Electron Beams from a Nanostructured Copper Cathode. Phys. Rev. Lett. 2013, 110, 074801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, M.; Ferguson, J.F.; Reiber, S.H. The pitting corrosion of copper. J. Am. Water Works Assoc. 1994, 86, 74–90. [Google Scholar] [CrossRef]
- Brusic, V. Copper Corrosion With and Without Inhibitors. J. Electrochem. Soc. 1991, 138, 2253. [Google Scholar] [CrossRef] [Green Version]
- Jeon, B.; Sankaranarayanan, S.K.R.S.; van Duin, A.C.T.; Ramanathan, S. Atomistic insights into aqueous corrosion of copper. J. Chem. Phys. 2011, 134, 234706. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Siow, K.-S.; Teo, W.-K.; Tan, K.-L.; Hsieh, A.-K. Corrosion Mechanisms and Products of Copper in Aqueous Solutions at Various pH Values. Corrosion 1997, 53, 389–398. [Google Scholar] [CrossRef]
- Pehkonen, S.O.; Palit, A.; Zhang, X. Effect of Specific Water Quality Parameters on Copper Corrosion. Corrosion 2002, 58, 156–165. [Google Scholar] [CrossRef]
- Kompfner, R. The Traveling-Wave Tube as Amplifier at Microwaves. Proc. IRE 1947, 35, 124–127. [Google Scholar] [CrossRef]
- Gill, C.; Wilczek, A. Crossed-Field Microwave Tube Having a Fluid Cooled Cathode and Control Electrode. U.S. Patent No. 3,612,932, 12 October 1971. [Google Scholar]
- Boyd, R.; Hendry, F.; Mannette, R. Fluid Cooled Traveling Wave Tube. U.S. Patent No. 3,246,190, 14 March 1966. [Google Scholar]
- Garven, M.; Calame, J.P.; Danly, B.G.; Nguyen, K.T.; Levush, B.; Wood, F.N.; Pershing, D.E. A gyrotron-traveling-wave tube amplifier experiment with a ceramic loaded interaction region. IEEE Trans. Plasma Sci. 2002, 30, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Calame, J.P.; Garven, M.; Danly, B.G.; Levush, B.; Nguyen, K.T. Gyrotron-traveling wave-tube circuits based on lossy ceramics. IEEE Trans. Electron Devices 2002, 49, 1469–1477. [Google Scholar] [CrossRef]
- Skowron, J.F. The continuous-cathode (emitting-sole) crossed-field amplifier. Proc. IEEE 1973, 61, 330–356. [Google Scholar] [CrossRef]
- MacPhail, G.R. Crossed-Field Amplifier. U.S. Patent No. 4,700,109, 13 October 1987. [Google Scholar]
- Okress, E.C. Crossed-Field Amplifier. U.S. Patent No. 3,082,351, 19 March 1963. [Google Scholar]
- McDougall, J.L.; McCall, L.; Stevenson, M.E. Water Chemistry and Processing Effects on the Corrosion Degradation of Copper Tubing in Cooling Water Systems. Pract. Fail. Anal. 2003, 3, 81–88. [Google Scholar] [CrossRef]
- Lee, H.P. Kinetics and Mechanisms of Cu Electrodissolution in Chloride Media. J. Electrochem. Soc. 1986, 133, 2035. [Google Scholar] [CrossRef]
- Braun, M.; Nobe, K. Electrodissolution Kinetics of Copper in Acidic Chloride Solutions. J. Electrochem. Soc. 1979, 126, 1666. [Google Scholar] [CrossRef]
- Bjorndahl, W.D.; Nobe, K. Copper Corrosion in Chloride Media. Effect of Oxygen. Corrosion 1984, 40, 82–87. [Google Scholar] [CrossRef]
- King, F.; Litke, C.D.; Quinn, M.J.; LeNeveu, D.M. The measurement and prediction of the corrosion potential of copper in chloride solutions as a function of oxygen concentration and mass-transfer coefficient. Corros. Sci. 1995, 37, 833–851. [Google Scholar] [CrossRef]
- Deslouis, C.; Tribollet, B.; Mengoli, G.; Musiani, M.M. Electrochemical behaviour of copper in neutral aerated chloride solution. I. Steady-state investigation. J. Appl. Electrochem. 1988, 18, 374–383. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.-M. Inhibition of Copper Corrosion in 3.0% NaCl Solution by N-Phenyl-1,4-phenylenediamine. J. Electrochem. Soc. 2005, 152, B428. [Google Scholar] [CrossRef]
- Eriksen, T.E.; Ndalamba, P.; Grenthe, I. On the corrosion of copper in pure water. Corros. Sci. 1989, 29, 1241–1250. [Google Scholar] [CrossRef]
- Hultquist, G. Why copper may be able to corrode in pure water. Corros. Sci. 2015, 93, 327–329. [Google Scholar] [CrossRef]
- Ottosson, M.; Boman, M.; Berastegui, P.; Andersson, Y.; Hahlin, M.; Korvela, M.; Berger, R. Copper in ultrapure water, a scientific issue under debate. Corros. Sci. 2017, 122, 53–60. [Google Scholar] [CrossRef]
- Hedin, A.; Johansson, A.J.; Lilja, C.; Boman, M.; Berastegui, P.; Berger, R.; Ottosson, M. Corrosion of copper in pure O2-free water? Corros. Sci. 2018, 137, 1–12. [Google Scholar] [CrossRef]
- Szakálos, P.; Hultquist, G.; Wikmark, G. Corrosion of Copper by Water. Electrochem. Solid-State Lett. 2007, 10, C63–C67. [Google Scholar] [CrossRef]
- Lu, L.; Shen, Y.; Chen, X.; Qian, L.; Lu, K. Ultrahigh Strength and High Electrical Conductivity in Copper. Science 2004, 304, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Parsons, G.N.; George, S.M.; Knez, M. Progress and future directions for atomic layer deposition and ALD-based chemistry. MRS Bull. 2011, 36, 865–871. [Google Scholar] [CrossRef] [Green Version]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Leskelä, M.; Ritala, M. Atomic Layer Deposition Chemistry: Recent Developments and Future Challenges. Angew. Chem. Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Meyer, J.; Riedl, T. Low-Temperature Atomic Layer Deposition. In Atomic Layer Deposition of Nanostructured Materials; Pinna, N., Knez, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 109–130. ISBN 9783527639915. [Google Scholar]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-Temperature Al2O3 Atomic Layer Deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Peng, Q.; Sun, X.-Y.; Spagnola, J.C.; Hyde, G.K.; Spontak, R.J.; Parsons, G.N. Atomic Layer Deposition on Electrospun Polymer Fibers as a Direct Route to Al2O3 Microtubes with Precise Wall Thickness Control. Nano Lett. 2007, 7, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Mundy, J.Z.; Shafiefarhood, A.; Li, F.; Khan, S.A.; Parsons, G.N. Low temperature platinum atomic layer deposition on nylon-6 for highly conductive and catalytic fiber mats. J. Vac. Sci. Technol. A 2016, 34, 01A152. [Google Scholar] [CrossRef]
- Brozena, A.H.; Oldham, C.J.; Parsons, G.N. Atomic layer deposition on polymer fibers and fabrics for multifunctional and electronic textiles. J. Vac. Sci. Technol. A 2016, 34, 010801. [Google Scholar] [CrossRef]
- Daubert, J.S.; Mundy, J.Z.; Parsons, G.N. Kevlar-Based Supercapacitor Fibers with Conformal Pseudocapacitive Metal Oxide and Metal Formed by ALD. Adv. Mater. Interfaces 2016, 3, 1600355. [Google Scholar] [CrossRef]
- Parsons, G.N.; Atanasov, S.E.; Dandley, E.C.; Devine, C.K.; Gong, B.; Jur, J.S.; Lee, K.; Oldham, C.J.; Peng, Q.; Spagnola, J.C.; et al. Mechanisms and reactions during atomic layer deposition on polymers. Coord. Chem. Rev. 2013, 257, 3323–3331. [Google Scholar] [CrossRef]
- Biercuk, M.J.; Monsma, D.J.; Marcus, C.M.; Becker, J.S.; Gordon, R.G. Low-temperature atomic-layer-deposition lift-off method for microelectronic and nanoelectronic applications. Appl. Phys. Lett. 2003, 83, 2405–2407. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.J.; Kwon, S.; Kim, H.; Park, J.-S. High performance thin film transistor with low temperature atomic layer deposition nitrogen-doped ZnO. Appl. Phys. Lett. 2007, 91, 183517. [Google Scholar] [CrossRef] [Green Version]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97. [Google Scholar] [CrossRef]
- Chang, M.L.; Cheng, T.C.; Lin, M.C.; Lin, H.C.; Chen, M.J. Improvement of oxidation resistance of copper by atomic layer deposition. Appl. Surf. Sci. 2012, 258, 10128–10134. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Ritala, M. Low-temperature atomic layer deposition of Al2O3thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2011, 53, 2168–2175. [Google Scholar] [CrossRef]
- Fedel, M.; Deflorian, F. Electrochemical characterization of atomic layer deposited Al2O3 coatings on AISI 316L stainless steel. Electrochim. Acta 2016, 203, 404–415. [Google Scholar] [CrossRef]
- Giorleo, L.; Ceretti, E.; Giardini, C. ALD coated tools in micro drilling of Ti sheet. CIRP Ann. 2011, 60, 595–598. [Google Scholar] [CrossRef]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Zanna, S.; Salmi, E.; Ritala, M.; Marcus, P. Corrosion protection of aluminium by ultra-thin atomic layer deposited alumina coatings. Corros. Sci. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vähä-Nissi, M.; Mustonen, T.; Iiskola, E.; Karppinen, M. Atomic layer deposited aluminum oxide barrier coatings for packaging materials. Thin Solid Films 2010, 518, 2654–2658. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vähä-Nissi, M.; Harlin, A.; Karppinen, M. Comparison of some coating techniques to fabricate barrier layers on packaging materials. Thin Solid Films 2010, 518, 5463–5466. [Google Scholar] [CrossRef]
- Lahtinen, K.; Maydannik, P.; Johansson, P.; Kääriäinen, T.; Cameron, D.C.; Kuusipalo, J. Utilisation of continuous atomic layer deposition process for barrier enhancement of extrusion-coated paper. Surf. Coat. Technol. 2011, 205, 3916–3922. [Google Scholar] [CrossRef]
- Kääriäinen, T.O.; Maydannik, P.; Cameron, D.C.; Lahtinen, K.; Johansson, P.; Kuusipalo, J. Atomic layer deposition on polymer based flexible packaging materials: Growth characteristics and diffusion barrier properties. Thin Solid Films 2011, 519, 3146–3154. [Google Scholar] [CrossRef]
- Bae, D.; Kwon, S.; Oh, J.; Kim, W.K.; Park, H. Investigation of Al2O3 diffusion barrier layer fabricated by atomic layer deposition for flexible Cu(In,Ga)Se2 solar cells. Renew. Energy 2013, 55, 62–68. [Google Scholar] [CrossRef]
- Baba Heidary, D.S.; Qu, W.; Randall, C.A. Evaluating the merit of ALD coating as a barrier against hydrogen degradation in capacitor components. RSC Adv. 2015, 5, 50869–50877. [Google Scholar] [CrossRef]
- Langereis, E.; Creatore, M.; Heil, S.B.S.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition of Al2O3 moisture permeation barriers on polymers. Appl. Phys. Lett. 2006, 89, 081915. [Google Scholar] [CrossRef]
- Park, S.K.; Oh, J.; Hwang, C.; Lee, J.; Yang, Y.S.; Chu, H.Y. Ultrathin Film Encapsulation of an OLED by ALD. Electrochem. Solid-State Lett. 2005, 8, H21. [Google Scholar] [CrossRef]
- Jarvis, K.L.; Evans, P.J. Growth of thin barrier films on flexible polymer substrates by atomic layer deposition. Thin Solid Films 2017, 624, 111–135. [Google Scholar] [CrossRef]
- Correa, G.C.; Bao, B.; Strandwitz, N.C. Chemical Stability of Titania and Alumina Thin Films Formed by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2015, 7, 14816–14821. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE: Houston, TX, USA, 1974; ISBN 978-0915567980. [Google Scholar]
- Sun, Q.; Yu, H.; Liu, Y.; Li, J.; Lu, Y.; Hunt, J.F. Improvement of water resistance and dimensional stability of wood through titanium dioxide coating. Holzforschung 2010, 64, 757–761. [Google Scholar] [CrossRef]
- Taruta, S.; Watanabe, K.; Kitajima, K.; Takusagawa, N. Effect of titania addition on crystallization process and some properties of calcium mica–apatite glass-ceramics. J. Non. Cryst. Solids 2003, 321, 96–102. [Google Scholar] [CrossRef]
- Karpagavalli, R.; Zhou, A.; Chellamuthu, P.; Nguyen, K. Corrosion behavior and biocompatibility of nanostructured TiO2 film on Ti6Al4V. J. Biomed. Mater. Res. Part A 2007, 83A, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Abdulagatov, A.I.; Yan, Y.; Cooper, J.R.; Zhang, Y.; Gibbs, Z.M.; Cavanagh, A.S.; Yang, R.G.; Lee, Y.C.; George, S.M. Al2O3 and TiO2 Atomic Layer Deposition on Copper for Water Corrosion Resistance. ACS Appl. Mater. Interfaces 2011, 3, 4593–4601. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Leygraf, C.; Thierry, D.; Ektessabi, A.M. Corrosion resistance for biomaterial applications of TiO2 films deposited on titanium and stainless steel by ion-beam-assisted sputtering. J. Biomed. Mater. Res. 1997, 35, 309–318. [Google Scholar] [CrossRef]
- Marin, E.; Guzman, L.; Lanzutti, A.; Ensinger, W.; Fedrizzi, L. Multilayer Al2O3/TiO2 Atomic Layer Deposition coatings for the corrosion protection of stainless steel. Thin Solid Films 2012, 522, 283–288. [Google Scholar] [CrossRef]
- Matero, R.; Ritala, M.; Leskelä, M.; Salo, T.; Aromaa, J.; Forsén, O. Atomic layer deposited thin films for corrosion protection. Le J. Phys. IV 1999, 9, 493–499. [Google Scholar] [CrossRef]
- Ćurković, L.; Ćurković, H.O.; Salopek, S.; Renjo, M.M.; Šegota, S. Enhancement of corrosion protection of AISI 304 stainless steel by nanostructured sol–gel TiO2 films. Corros. Sci. 2013, 77, 176–184. [Google Scholar] [CrossRef]
- Li, S.; Fu, J. Improvement in corrosion protection properties of TiO2 coatings by chromium doping. Corros. Sci. 2013, 68, 101–110. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion Protection of Copper Using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Klein, L.H.; Härkönen, E.; Ritala, M.; Marcus, P. Electrochemical and Surface Analysis of the Corrosion Protection of Copper by Nanometer-Thick Alumina Coatings Prepared by Atomic Layer Deposition. J. Electrochem. Soc. 2015, 162, C377–C384. [Google Scholar] [CrossRef]
- Chai, Z.; Li, J.; Lu, X.; He, D. Use of electrochemical measurements to investigate the porosity of ultra-thin Al2O3 films prepared by atomic layer deposition. RSC Adv. 2014, 4, 39365–39371. [Google Scholar] [CrossRef]
- Chai, Z.; Liu, Y.; Li, J.; Lu, X.; He, D. Ultra-thin Al2O3 films grown by atomic layer deposition for corrosion protection of copper. RSC Adv. 2014, 4, 50503–50509. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Guzman, L.; Fedrizzi, L. Chemical and electrochemical characterization of TiO2/Al2O3 atomic layer depositions on AZ-31 magnesium alloy. J. Coat. Technol. Res. 2012, 9, 347–355. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Lekka, M.; Guzman, L.; Ensinger, W.; Fedrizzi, L. Chemical and mechanical characterization of TiO2/Al2O3 atomic layer depositions on AISI 316 L stainless steel. Surf. Coat. Technol. 2012, 211, 84–88. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part I. Establishment of equivalent circuits for EIS data modelling. Corros. Sci. 2003, 45, 1243–1256. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef] [Green Version]
- Láng, G.; Heusler, K.E. Remarks on the energetics of interfaces exhibiting constant phase element behaviour. J. Electroanal. Chem. 1998, 457, 257–260. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Maurice, V.; Światowska, J.; Seyeux, A.; Ritala, M.; Marcus, P. Failure mechanism of thin Al2O3 coatings grown by atomic layer deposition for corrosion protection of carbon steel. Electrochim. Acta 2011, 56, 9609–9618. [Google Scholar] [CrossRef]
- Bianchi, G.; Fiori, G.; Longhi, P.; Mazza, F. “Horse Shoe” Corrosion of Copper Alloys in Flowing Sea Water: Mechanism, and Possibility of Cathodic Protection of Condenser Tubes in Power Stations. Corrosion 1978, 34, 396–406. [Google Scholar] [CrossRef]
- Vazquez, M.V.; de Sanchez, S.R.; Calvo, E.J.; Schiffrin, D.J. The electrochemical reduction of oxygen on polycrystalline copper in borax buffer. J. Electroanal. Chem. 1994, 374, 189–197. [Google Scholar] [CrossRef]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Klein, L.H.; Salmi, E.; Ritala, M.; Marcus, P. Interfacial native oxide effects on the corrosion protection of copper coated with ALD alumina. Electrochim. Acta 2016, 193, 7–15. [Google Scholar] [CrossRef]
- Van Hemmen, J.L.; Heil, S.B.S.; Klootwijk, J.H.; Roozeboom, F.; Hodson, C.J.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma and Thermal ALD of Al2O3 in a Commercial 200 mm ALD Reactor. J. Electrochem. Soc. 2007, 154, G165. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Jakschik, S.; Schroeder, U.; Hecht, T.; Gutsche, M.; Seidl, H.; Bartha, J.W. Crystallization behavior of thin ALD- Al2O3 films. Thin Solid Films 2003, 425, 216–220. [Google Scholar] [CrossRef]
- Härkönen, E.; Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Vehkamäki, M.; Sajavaara, T.; Fenker, M.; Marcus, P.; Ritala, M. Corrosion Protection of Steel with Oxide Nanolaminates Grown by Atomic Layer Deposition. J. Electrochem. Soc. 2011, 158, C369. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mändar, H.; Uustare, T.; Schuisky, M.; Hårsta, A. Atomic layer growth of epitaxial TiO2 thin films from TiCl4 and H2O on α-Al2O3 substrates. J. Cryst. Growth 2002, 242, 189–198. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Kiisler, A.; Uustare, T.; Sammelselg, V. Effect of crystal structure on optical properties of TiO2 films grown by atomic layer deposition. Thin Solid Films 1997, 305, 270–273. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Uustare, T.; Sammelselg, V. Morphology and structure of TiO2 thin films grown by atomic layer deposition. J. Cryst. Growth 1995, 148, 268–275. [Google Scholar] [CrossRef]
- Aarik, J.; Aidla, A.; Mändar, H.; Uustare, T. Atomic layer deposition of titanium dioxide from TiCl4 and H2O: Investigation of growth mechanism. Appl. Surf. Sci. 2001, 172, 148–158. [Google Scholar] [CrossRef]
- Ritala, M.; Leskelä, M.; Nykänen, E.; Soininen, P.; Niinistö, L. Growth of titanium dioxide thin films by atomic layer epitaxy. Thin Solid Films 1993, 225, 288–295. [Google Scholar] [CrossRef]














| Sample Name | Nominal Film Structure | Si Thickness (nm) | Cu Thickness (nm) | Refractive Index |
|---|---|---|---|---|
| Cu | None—uncoated copper | - | - | - |
| Al2O3 | Single-layer Al2O3 | 52.9 ± 0.6 | 49.1 ± 0.7 | 1.64 |
| TiO2 | Single-layer TiO2 | 54.0 ± 1.6 | 63.8 ± 2.8 | 2.43 |
| ATx10 | Nanolaminate—(Al2O3 + TiO2) × 10 | 56.0 ± 0.6 | 65.8 ± 2.5 | 2.1 |
| ATx5 | Nanolaminate—(Al2O3 + TiO2) × 5 | 52.5 ± 0.8 | 56.6 ± 1.9 | 2.12 |
| ATx1 | Double layer—10 nm Al2O3 + 40 nm TiO2 | 52.6 ± 0.9 | 60.9 ± 1.1 | 2.33 |
| Sample | Deoxygenated | Oxygenated | ||||
|---|---|---|---|---|---|---|
| Ecorr (V vs. Ag/AgCl) | Rp (Ω cm2) × 106 | Icorr (A cm−2) × 10−7 | Ecorr (V vs. Ag/AgCl) | Rp (Ω cm2) × 106 | Icorr (A cm−2) × 10−7 | |
| Cu | −0.347 | 0.191 | 1.51 | −0.355 | 0.066 | 3.77 |
| Al2O3 | −0.395 | 1.67 | 0.179 | −0.314 | 0.204 | 1.07 |
| TiO2 | −0.314 | 0.373 | 0.915 | −0.379 | 0.193 | 0.992 |
| ATx1 | −0.328 | 3.37 | 0.103 | −0.318 | 0.948 | 0.167 |
| ATx5 | −0.350 | 2.60 | 0.115 | −0.349 | 0.596 | 0.340 |
| ATx10 | −0.444 | 1.85 | 0.197 | −0.325 | 0.167 | 1.09 |
| Deoxygenated | ||||||||
| Rs (Ω cm2) | Qfilm (Ω−1 sa cm−2) | αfilm | Rfilm (Ω cm2) | Qint (Ω−1 sa cm−2) | αint | Rint (Ω cm2) | ||
| Cu | 124.7 | 2.08 × 10−5 | 0.87 | 6831 | 3.14 × 10−5 | 0.62 | 1.23 × 105 | |
| Al2O3 | 120.1 | 2.65 × 10−6 | 0.74 | 5320 | 2.04 × 10−5 | 0.65 | 6.30 × 106 | |
| TiO2 | 116.5 | 5.63 × 10−6 | 0.93 | 4759 | 4.83 × 10−5 | 0.80 | 1.48 × 106 | |
| ATx1 | 130.2 | 9.05 × 10−7 | 0.91 | 11,642 | 1.54 × 10−6 | 0.4 | 3.97 × 106 | |
| ATx5 | 126.2 | 4.43 × 10−7 | 0.98 | 18,858 | 1.45 × 10−6 | 0.63 | 1.11 × 107 | |
| ATx10 | 126.3 | 3.75 × 10v6 | 0.79 | 343.5 | 2.08 × 10−5 | 0.86 | 5.19 × 106 | |
| Oxygenated | ||||||||
| Rs (Ω cm2) | Qfilm (Ω−1 sa cm−2) | αfilm | Rfilm (Ω cm2) | Qint (Ω−1 sa cm−2) | αint | Rint (Ω cm2) | Wint (Ω s−0.5 cm2) | |
| Cu | 135.6 | 8.08 × 10−5 | 0.90 | 272.5 | 1.46 × 10−4 | 0.58 | 2.18 × 105 | 2.2 × 10−9 |
| Al2O3 | 116.8 | 3.02 × 10−6 | 0.74 | 385 | 8.96 × 10−5 | 0.73 | 7.60 × 105 | 4.8 × 10−10 |
| TiO2 | 126 | 7.16 × 10−6 | 0.86 | 5324 | 3.81 × 10−5 | 0.58 | 4.85 × 105 | 9.2 × 10−10 |
| ATx1 | 128 | 8.03 × 10−7 | 0.91 | 65,445 | 1.26 × 10−6 | 0.77 | 3.02 × 105 | 1.6 × 104 |
| ATx5 | 123.9 | 4.58 × 10−7 | 0.97 | 72,013 | 1.96 × 10−6 | 0.64 | 5.50 × 106 | 1.6 × 10−3 |
| ATx10 | 134.3 | 3.35 × 10−7 | 0.99 | 24,467 | 2.63 × 10−6 | 0.42 | 4.58 × 106 | 4.4 × 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, M.A.; Oldham, C.J.; Parsons, G.N. Investigation of the Corrosion Behavior of Atomic Layer Deposited Al2O3/TiO2 Nanolaminate Thin Films on Copper in 0.1 M NaCl. Materials 2019, 12, 672. https://doi.org/10.3390/ma12040672
Fusco MA, Oldham CJ, Parsons GN. Investigation of the Corrosion Behavior of Atomic Layer Deposited Al2O3/TiO2 Nanolaminate Thin Films on Copper in 0.1 M NaCl. Materials. 2019; 12(4):672. https://doi.org/10.3390/ma12040672
Chicago/Turabian StyleFusco, Michael A., Christopher J. Oldham, and Gregory N. Parsons. 2019. "Investigation of the Corrosion Behavior of Atomic Layer Deposited Al2O3/TiO2 Nanolaminate Thin Films on Copper in 0.1 M NaCl" Materials 12, no. 4: 672. https://doi.org/10.3390/ma12040672
APA StyleFusco, M. A., Oldham, C. J., & Parsons, G. N. (2019). Investigation of the Corrosion Behavior of Atomic Layer Deposited Al2O3/TiO2 Nanolaminate Thin Films on Copper in 0.1 M NaCl. Materials, 12(4), 672. https://doi.org/10.3390/ma12040672