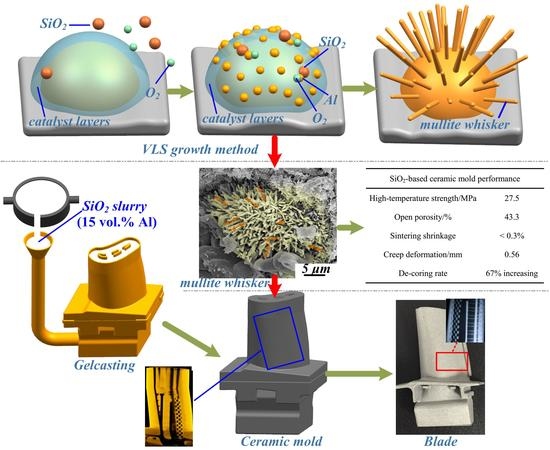
Effect of Urchin-Like Mullite Whiskers on the High-Temperature Performance of Porous SiO2-Based Ceramic Molds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Gelcasting Process
2.3. Measurements
3. Results and Discussion
3.1. Synthesis of Mullite Whiskers
3.1.1. VLS Growth Mechanism
3.1.2. Thermal Analysis
3.2. Microstructure Analysis
3.3. High-Temperature Performance Analysis
3.4. Case Study
4. Conclusions
- (1)
- Polycrystalline urchin-like mullite whiskers were successfully synthesized through the VLS growth method. Urchin-like mullite whiskers were significantly effective in improving the high-temperature strength of SiO2-based ceramic molds through the bridge effect. The SiO2-based ceramic mold obtained a maximum bending strength of 27.5 MPa with a 15 vol % Al additive.
- (2)
- When 15 vol% Al was added to the raw material, a minimum creep deformation of 0.56 mm and a sintering shrinkage below 0.3% were obtained, and the de-coring rate increased by 67%. An integral ceramic mold for a hollow turbine blade was successfully fabricated.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chao, C.H.; Lu, H.Y. Optimal composition of zircon–fused silica ceramic cores for casting superalloys. J. Am. Ceram. Soc. 2002, 85, 773–779. [Google Scholar] [CrossRef]
- Mishra, S.; Mitra, R.; Vijayakumar, M. Structure–property correlation in cellular silica processed through hydrophobized fused silica powder for aerospace application. J. Alloy. Compd. 2010, 504, 76–82. [Google Scholar] [CrossRef]
- Han, J.; Hu, L.; Zhang, Y.; Jiang, Z.; Zhou, Y. In situ synthesis of hierarchically porous silica ceramics with unidirectionally aligned channel structure. Scr. Mater. 2010, 62, 431–434. [Google Scholar] [CrossRef]
- Wan, W.; Yang, J.; Zeng, J.; Yao, L.; Qiu, T. Effect of solid loading on gelcasting of silica ceramics using DMAA. Ceram. Int. 2014, 40, 1735–1740. [Google Scholar] [CrossRef]
- Wu, H.; Li, D.; Guo, N. Fabrication of integral ceramic mold for investment casting of hollow turbine blade based on stereolithography. Rapid Prototyp. J. 2009, 15, 232–237. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Y.; Miao, K.; Xu, W.; Liu, T.; Yang, Q.; Li, D. Microstructures and high-temperature strength of gel-casting Al2O3-based ceramic molds with coated aluminum additive. Int. J. Adv. Manuf. Technol. 2017, 94, 845–854. [Google Scholar] [CrossRef]
- Lu, Z.; Tian, G.; Wan, W.; Miao, K.; Li, D. Effect of in situ synthesised mullite whiskers on the high-temperature strength of Al2O3-based ceramic moulds for casting hollow turbine blades. Ceram. Int. 2016, 42, 18851–18858. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yeo, J.G.; Lee, J.S.; Choi, S.C. Influence of silicon carbide as a mineralizer on mechanical and thermal properties of silica-based ceramic cores. Ceram. Int. 2016, 42, 14738–14742. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, L.; Zhang, L.; Hua, Y.; Yang, W. Microstructure and properties of particle reinforced silicon carbide and silicon nitride ceramic matrix composites prepared by chemical vapor infiltration. Mater. Sci. Eng. A 2008, 475, 217–223. [Google Scholar] [CrossRef]
- Jones, S.; Yuan, C. Advances in shell moulding for investment casting. J. Mater. Process Technol. 2003, 135, 258–265. [Google Scholar] [CrossRef]
- Gao, H.T.; Liu, X.H.; Chen, J.Q.; Qi, J.L.; Wang, Y.B.; Ai, Z.R. Preparation of glass-ceramics with low density and high strength using blast furnace slag, glass fiber and water glass. Ceram. Int. 2018, 44, 6044–6053. [Google Scholar] [CrossRef]
- Deng, Y.; Li, W.; Wang, X.; Kou, H.; Zhang, X.; Shao, J.; Li, Y.; Zhang, X.; Ma, J.; Tao, Y.; et al. Temperature-dependent tensile strength model for 2D woven fiber reinforced ceramic matrix composites. J. Am. Ceram. Soc. 2018, 101, 5157–5165. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Yang, J.; Zhang, W.; Wang, Y. Sintering Behavior and Mechanical Properties of Mullite Fibers/Hydroxyapatite Ceramic. Materials 2018, 11, 1859. [Google Scholar] [CrossRef]
- Miyake, K.; Hirata, Y.; Shimonosono, T.; Sameshima, S. The Effect of Particle Shape on Sintering Behavior and Compressive Strength of Porous Alumina. Materials 2018, 11, 1137. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, J.G. Synthesis of mullite whiskers. J. Am. Ceram. Soc. 2002, 85, 481–483. [Google Scholar] [CrossRef]
- Meng, B.; Peng, J. Effects of in situ synthesized mullite whiskers on flexural strength and fracture toughness of corundum-mullite refractory materials. Ceram. Int. 2013, 39, 1525–1531. [Google Scholar] [CrossRef]
- Peng, P.; Sorrell, C. Preparation of mullite whiskers from topaz decomposition. Mater. Lett. 2004, 58, 1288–1291. [Google Scholar] [CrossRef]
- Park, Y.M.; Yang, T.Y.; Yoon, S.Y.; Stevens, R.; Park, H.C. Mullite whiskers derived from coal fly ash. Mater. Sci. Eng. A 2007, 454–455, 518–522. [Google Scholar] [CrossRef]
- Li, K.Q.; Shimizu, T.D.; Igarashi, K.R. Preparation of Short Mullite Fibers from Kaolin via the Addition of Foaming Agents. J. Am. Ceram. Soc. 2001, 84, 497–503. [Google Scholar] [CrossRef]
- Mao, Y.B.; Kanungo, M.; Hemraj-Benny, T.; Wong, S.S. synthesis and growth mechanism of titanate and titania one-dimensional nanostructures self-assembled into Hollow micrometer-scale spherical aggregates. J. Phys. Chem. B 2006, 110, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, L.; Liu, L.; Li, J.; Yu, H.; Li, W.; Chen, Y. Microstructure and mechanical properties of hot-pressed Al2O3–mullite–ZrO2–SiC composites. Mater. Sci. Eng. A 2019, 740–741, 390–397. [Google Scholar] [CrossRef]
- Yu, P.C.; Tsai, Y.W.; Yen, F.S.; Huang, C.L.; Wei, W.C. Thermal reaction of cristobalite in nano-SiO2/α-Al2O3 powder systems for mullite synthesis. J. Am. Ceram. Soc. 2014, 97, 2431–2438. [Google Scholar] [CrossRef]
- Yu, P.C.; Tsai, Y.W.; Yen, F.S.; Yang, W.P.; Huang, C.L. Thermal characteristic difference between α-Al2O3 and cristobalite powders during mullite synthesis induced by size reduction. J. Eur. Ceram. Soc. 2015, 35, 673–680. [Google Scholar] [CrossRef]
- Wu, S.X.; Holz, D.; Claussen, N. Mechanisms and Kinetics of Reaction-Bonded. J. Eur. Ceram. Soc. 1993, 76, 970–980. [Google Scholar] [CrossRef]
- Lu, Z.; Miao, K.; Zhu, W.; Chen, Y.; Xia, Y.; Li, D. Fractions design of irregular particles in suspensions for the fabrication of multiscale ceramic components by gelcasting. J. Eur. Ceram. Soc. 2018, 38, 671–678. [Google Scholar] [CrossRef]
- Tian, G.; Lu, Z.; Miao, K.; Ji, Z.; Zhang, H.; Li, D.; Lloyd, I. Formation Mechanism of Cracks During the Freeze Drying of Gelcast Ceramic Parts. J. Am. Ceram. Soc. 2015, 98, 3338–3345. [Google Scholar] [CrossRef]
- Tkalcec, E.; Ivankovic, H.; Nass, R.; Schmidt, H. Crystallization kinetics of mullite formation in diphasic gels containing different alumina components. J. Eur. Ceram. Soc. 2003, 23, 1465–1475. [Google Scholar] [CrossRef]
- Miao, K.; Lu, Z.; Cao, J.; Zhang, H.; Li, D. Effect of polydimethylsiloxane on the mid-temperature strength of gelcast Al2O3 ceramic parts. Mater. Des. 2016, 89, 810–814. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Huang, Y. Recent developments in gelcasting of ceramics. J. Eur. Ceram. Soc. 2011, 31, 2569–2591. [Google Scholar] [CrossRef]
- Ortega, F.S.; Valenzuela, F.A.O.; Scuracchio, C.H.; Pandolfelli, V.C. Alternative gelling agents for the gelcasting of ceramic foams. J. Eur. Ceram. Soc. 2003, 23, 75–80. [Google Scholar] [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- Montanaro, L.; Coppola, B.; Palmero, P.; Tulliani, J.-M. A review on aqueous gelcasting: A versatile and low-toxic technique to shape ceramics. Ceram. Int. 2019, 45, 9653–9673. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Z.; Ji, Z.; Li, D. Basis for the alkaline removal process design of the alumina-based ceramic core. J. Ceram. Soc. Jpn. 2017, 125, 616–622. [Google Scholar] [CrossRef] [Green Version]










| Sample | No. Weight Fraction (vol %) | ||||
|---|---|---|---|---|---|
| SiO2 (100 µm) | SiO2 (40 µm) | SiO2 (5 µm) | SiO2 (2 µm) | Al (40 µm) | |
| S1 | 22.1 | 40.9 | 21.6 | 14.4 | 0 |
| S2 | 22.1 | 35.9 | 21.6 | 14.4 | 5.0 |
| S3 | 22.1 | 30.9 | 21.6 | 14.4 | 10.0 |
| S4 | 22.1 | 25.9 | 21.6 | 14.4 | 15.0 |
| S5 | 22.1 | 20.9 | 21.6 | 14.4 | 20.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lu, Z.; Wan, W.; Li, J.; Miao, K.; Li, D. Effect of Urchin-Like Mullite Whiskers on the High-Temperature Performance of Porous SiO2-Based Ceramic Molds. Materials 2019, 12, 1181. https://doi.org/10.3390/ma12071181
Chen Y, Lu Z, Wan W, Li J, Miao K, Li D. Effect of Urchin-Like Mullite Whiskers on the High-Temperature Performance of Porous SiO2-Based Ceramic Molds. Materials. 2019; 12(7):1181. https://doi.org/10.3390/ma12071181
Chicago/Turabian StyleChen, Yi, Zhongliang Lu, Weijian Wan, Jian Li, Kai Miao, and Dichen Li. 2019. "Effect of Urchin-Like Mullite Whiskers on the High-Temperature Performance of Porous SiO2-Based Ceramic Molds" Materials 12, no. 7: 1181. https://doi.org/10.3390/ma12071181
APA StyleChen, Y., Lu, Z., Wan, W., Li, J., Miao, K., & Li, D. (2019). Effect of Urchin-Like Mullite Whiskers on the High-Temperature Performance of Porous SiO2-Based Ceramic Molds. Materials, 12(7), 1181. https://doi.org/10.3390/ma12071181