Synthesis, Optical, and Morphological Studies of ZnO Powders and Thin Films Fabricated by Wet Chemical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
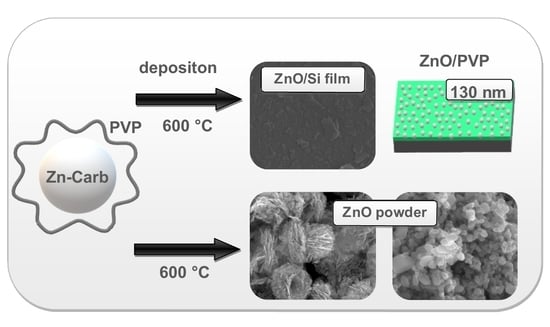
2.2. Synthesis of ZnO Powders
2.3. Thin Layer Fabrication
2.4. Characterization Methods
3. Results and Discussion
3.1. Synthesis and Characterization of Powders
3.2. Thin Layer Fabrication and Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, 829–858. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials (Basel) 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Groenen, R.; Linden, J.L.; Van Lierop, H.R.M.; Schram, D.C.; Kuypers, A.D.; Van De Sanden, M.C.M. Expanding thermal plasma for deposition of surface textured ZnO: Al with focus on thin film solar cell applications. Appl. Surf. Sci. 2001, 173, 40–43. [Google Scholar] [CrossRef]
- Gordillo, G.; Calderón, C. Properties of ZnO thin films prepared by reactive evaporation. Sol. Energy Mater. Sol. Cells 2001, 69, 251–260. [Google Scholar] [CrossRef]
- Lupan, O.; Shishiyanu, S.; Ursaki, V.; Khallaf, H.; Chow, L.; Shishiyanu, T.; Sontea, V.; Monaico, E.; Railean, S. Synthesis of nanostructured Al-doped zinc oxide films on Si for solar cells applications. Sol. Energy Mater. Sol. Cells 2009, 93, 1417–1422. [Google Scholar] [CrossRef]
- Qiao, S.; Liu, J.; Fu, G.; Ren, K.; Li, Z.; Wang, S.; Pan, C. ZnO nanowire based CIGS solar cell and its efficiency enhancement by the piezo-phototronic effect. Nano Energy 2018, 49, 508–514. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.; Wang, Z.L. Dissolving behavior and stability of ZnO wires in biofluids: A study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Ludi, B.; Niederberger, M. Zinc oxide nanoparticles: Chemical mechanisms and classical and non-classical crystallization. Dalt. Trans. 2013, 42, 12554–12568. [Google Scholar] [CrossRef]
- Fang, X.; Bando, Y.; Gautam, U.K.; Zhai, T.; Zeng, H.; Xu, X.; Liao, M.; Golberg, D. ZnO and ZnS nanostructures: Ultraviolet-light emitters, lasers, and sensors. Crit. Rev. Solid State Mater. Sci. 2009, 34, 190–223. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Soares, N.F.F.; dos Coimbra, J.S.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Jun, M.C.; Park, S.U.; Koh, J.H. Comparative studies of Al-doped ZnO and Gadoped ZnO transparent conducting oxide thin films. Nanoscale Res. Lett. 2012, 7, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abed, S.; Bougharraf, H.; Bouchouit, K.; Sofiani, Z.; Derkowska-Zielinska, B.; Aida, M.S.; Sahraoui, B. Influence of Bi doping on the electrical and optical properties of ZnO thin films. Superlattices Microstruct. 2015, 85, 370–378. [Google Scholar] [CrossRef]
- Salles, T.H.C.; Lombello, C.B.; D’Ávila, M.A. Electrospinning of gelatin/poly (vinyl pyrrolidone) blends from water/acetic acid solutions. Mater. Res. 2015, 18, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Wang, Q.; Zhang, M.; Deng, Q.; Chen, D. Facile fabrication of raspberry-like composite microspheres for the construction of superhydrophobic films and applications in highly efficient oil-water separation. RSC Adv. 2017, 7, 39471–39479. [Google Scholar] [CrossRef] [Green Version]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Ilegbusi, O.J.; Song, H.; Chakrabarti, R. Biocompatibility and Conductometric Property of Sol-Gel Derived ZnO/PVP Nanocomposite Biosensor Film. J. Bionic Eng. 2010, 7, 30–35. [Google Scholar] [CrossRef]
- Du, T.; Song, H.; Ilegbusi, O.J. Sol-gel derived ZnO/PVP nanocomposite thin film for superoxide radical sensor. Mater. Sci. Eng. C 2007, 27, 414–420. [Google Scholar] [CrossRef]
- Kim, O.S.; Kwon, J.B.; Kim, S.W.; Xu, B.; Seo, K.H.; Park, C.E.; Do, W.J.; Bae, J.H.; Kang, S.W. Effect of PVP-Capped ZnO Nanoparticles with Enhanced Charge Transport on the Performance of P3HT/PCBM Polymer Solar Cells. Polymers (Basel) 2019, 11, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudebs, G.; Besse, V.; Cassagne, C.; Leblond, H.; de Araújo, C.B. Nonlinear characterization of materials using the D4σ method inside a Z-scan 4f-system. Opt. Lett. 2013, 38, 2206. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, C.B.; Gomes, A.S.L.; Boudebs, G. Techniques for nonlinear optical characterization of materials: A review. Rep. Prog. Phys. 2016, 79, 036401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; Van Stryland, E.W. Sensitive Measurement of Optical Nonlinearities Using a Single Beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Derkowska-Zielinska, B.; Fedus, K.; Wang, H.; Cassagne, C.; Boudebs, G. Nonlinear optical characterization of Disperse Orange 3. Opt. Mater. (Amst.) 2017, 72, 545–548. [Google Scholar] [CrossRef]
- Fedus, K.; Boudebs, G. Experimental techniques using 4f coherent imaging system for measuring nonlinear refraction. Opt. Commun. 2013, 292, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Gnoli, A.; Razzari, L.; Righini, M. Z-scan measurements using high repetition rate lasers: How to manage thermal effects. Opt. Express 2005, 13, 7976–7981. [Google Scholar] [CrossRef]
- Hales, M.C.; Frost, R.L. Synthesis and vibrational spectroscopic characterisation of synthetic hydrozincite and smithsonite. Polyhedron 2007, 26, 4955–4962. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Poduska, K. A Strategy for Hydroxide Exclusion in Nanocrystalline Solid-State Metathesis Products. Nanomaterials 2013, 3, 317–324. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Saion, E.B.; Shaari, A.H.; Kamarudin, M.A.; Flaifel, M.H.; Ahmad, S.H.; Gene, S.A. A facile thermal-treatment route to synthesize ZnO nanosheets and effect of calcination temperature. PLoS ONE 2014, 9, e103134. [Google Scholar] [CrossRef]
- Soltani, N.; Saion, E.; Erfani, M.; Rezaee, K.; Bahmanrokh, G.; Drummen, G.P.C.; Bahrami, A.; Hussein, M.Z. Influence of the polyvinyl pyrrolidone concentration on particle size and dispersion of ZnS nanoparticles synthesized by microwave irradiation. Int. J. Mol. Sci. 2012, 13, 12412–12427. [Google Scholar] [CrossRef] [PubMed]
- Kamari, H.; Naseri, M.; Saion, E. A Novel Research on Behavior of Zinc Ferrite Nanoparticles in Different Concentration of Poly(vinyl pyrrolidone) (PVP). Metals (Basel) 2014, 4, 118–129. [Google Scholar] [CrossRef]
- Wang, X.; Cai, W.; Lin, Y.; Wang, G.; Liang, C. Mass production of micro/nanostructured porous ZnO plates and their strong structurally enhanced and selective adsorption performance for environmental remediation. J. Mater. Chem. 2010, 20, 8582–8590. [Google Scholar] [CrossRef]
- Gasaymeh, S.S.; Radiman, S.; Heng, L.Y.; Saion, E.; Mohamed Saeed, G.H. Synthesis and characterization of silver/Polyvinilpirrolidone (AG/PVP) nanoparticles using gamma irradiation techniques. Am. J. Appl. Sci. 2010, 7, 879–888. [Google Scholar] [CrossRef]
- Han, W.; Yang, K.; Li, D.; Zhang, Z.; Ma, J.; Ni, S.; Yang, X. The fabrication and characterization of Zn5(CO3)2(OH)6 as a new anode material for lithium ion batteries. Mater. Lett. 2016, 164, 148–151. [Google Scholar] [CrossRef]
- Jing, Z.; Zhan, J. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008, 20, 4547–4551. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and photoluminescence characterization of ZnO nanoparticles. J. Lumin. 2013, 134, 213–219. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Xu, M.; Wang, Y.; Zhu, B.; Zhang, S.; Huang, W.; Wu, S. Hierarchically Porous ZnO Architectures for Gas Sensor Application. Cryst. Growth Des. 2009, 9, 3532–3537. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Loudon, R. The Raman effect in crystals. Adv. Phys. 1964, 13, 423–482. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakamori, N.; Kanamori, H.; Yodogawa, Y.; Yamamoto, K. Infrared study of surface phonon modes in ZnO, CdS and BeO small crystals. Surf. Sci. 1979, 86, 665–671. [Google Scholar] [CrossRef]
- Andrés-Vergés, M.; Serna, C.J. Morphological characterization of ZnO powders by X-ray and IR spectroscopy. J. Mater. Sci. Lett. 1988, 7, 970–972. [Google Scholar] [CrossRef]
- Herron, S.M.; Tanskanen, J.T.; Roelofs, K.E.; Bent, S.F. Highly textured tin(II) sulfide thin films formed from sheetlike nanocrystal inks. Chem. Mater. 2014, 26, 7106–7113. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Köferstein, R.; Jäger, L.; Ebbinghaus, S.G. Magnetic and optical investigations on LaFeO3 powders with different particle sizes and corresponding ceramics. Solid State Ion. 2013, 249–250, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Urbina, J.; Cruz-Alonso, A.; Santander-González, M.; Méndez-Albores, A.; Vázquez-Durán, A. Nanoscale Zinc Oxide Particles for Improving the Physiological and Sanitary Quality of a Mexican Landrace of Red Maize. Nanomaterials 2018, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic Growth of Zinc Oxide Nanowires by Vapor Transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Van Dijken, A.; Makkinje, J.; Meijerink, A. The influence of particle size on the luminescence quantum efficiency of nanocrystalline ZnO particles. J. Lumin. 2001, 92, 323–328. [Google Scholar] [CrossRef]
- Fonoberov, V.A.; Alim, K.A.; Balandin, A.A.; Xiu, F.; Liu, J. Photoluminescence investigation of the carrier recombination processes in ZnO quantum dots and nanocrystals. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 73, 165317. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Fan, H.; Ma, Y.; Wang, Z.; Chang, Q. Surface defects control for ZnO nanorods synthesized by quenching and their anti-recombination in photocatalysis. Appl. Surf. Sci. 2015, 332, 47–54. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van De Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ciret, C.; Cassagne, C.; Boudebs, G. Measurement of the third order optical nonlinearities of graphene quantum dots in water at 355 nm, 532 nm and 1064 nm. Opt. Mater. Express 2019, 9, 339. [Google Scholar] [CrossRef]
- J.A. Woolam Co. Inc. Guide to Using WVASE32®; Wextech Syst. Inc.: New York, NY, USA, 2010. [Google Scholar]
- Fujiwara, H. Spectroscopic Ellipsometry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780470016084. [Google Scholar]
- Skowronski, L.; Wachowiak, A.A.; Grabowski, A. Characterization of optical and microstructural properties of semitransparent TiO2 /Ti/glass interference decorative coatings. Appl. Surf. Sci. 2016, 388, 731–740. [Google Scholar] [CrossRef]
- Skowronski, L.; Wachowiak, A.A.; Wachowiak, W. Optical and microstructural properties of decorative Al/Ti/TiO2 interference coatings. Appl. Surf. Sci. 2017, 421, 794–801. [Google Scholar] [CrossRef]
- Skowronski, L.; Wachowiak, A.A.; Zdunek, K.; Trzcinski, M.; Naparty, M.K. TiO2-based decorative coatings deposited on the AISI 316L stainless steel and glass using an industrial scale magnetron. Thin Solid Films 2017, 627, 1–8. [Google Scholar] [CrossRef]
- Lejnieks, J.; Mourran, A.; Tillmann, W.; Keul, H.; Möller, M. Thin film of Poly(acrylic acid-co-allyl acrylate) as a Sacrificial Protective Layer for Hydrophilic Self Cleaning Glass. Materials (Basel) 2010, 3, 3369–3384. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Chang, S.I.; Yoon, E. A flexible polymer tactile sensor: Fabrication and modular expandability for large area deployment. J. Microelectromech. Syst. 2006, 15, 1681–1686. [Google Scholar] [CrossRef]
- Calleja, A.; Ricart, S.; Aklalouch, M.; Mestres, N.; Puig, T.; Obradors, X. Thickness–concentration–viscosity relationships in spin-coated metalorganic ceria films containing polyvinylpyrrolidone. J. Sol-Gel Sci. Technol. 2014, 72, 21–29. [Google Scholar] [CrossRef]
- Lampande, R.; Kim, G.W.; Pode, R.; Kwon, J.H. Effectiveness of a polyvinylpyrrolidone interlayer on a zinc oxide film for interfacial modification in inverted polymer solar cells. RSC Adv. 2014, 4, 49855–49860. [Google Scholar] [CrossRef]















| Sample | α (cm−1) | E (µJ) | I0 (GW/cm2) | n2 × 10−20 (m2/W) | β (cm/GW) |
|---|---|---|---|---|---|
| chloroform | 0 | 15 | 92 | 6.25 ± 1.0 | < 0.004 |
| A-type | 0.42 | 15 | 95 | 4.6 ± 1.0 | < 0.004 |
| B-type | 1.20 | 15 | 92 | 6.5 ± 1.3 | < 0.004 |
| Sample | α (cm−1) | E (µJ) | I0 (GW/cm2) | n2 × 10−20 (m2/W) | β (cm/GW) |
|---|---|---|---|---|---|
| chloroform | 0 | 5 | 65 | 7.5 ± 2.6 | 0.15 ± 0.05 |
| A-type | 0.59 | 5 | 64 | 5.5 ± 2.5 | 0.08 ± 0.02 |
| B-type | 2.12 | 5 | 66 | 9.6 ± 2.7 | 0.31 ± 0.10 |
| No. | PVP Concentration (%) | Spin-Coating Parameters | Film Thickness (nm) | Thickness Non-Uniformity (%) |
|---|---|---|---|---|
| 1 | 2.5 | Step 1: 5000 rpm 30 s Step 2: 5000 rpm 30 s | 133 ± 1 | 13.1 ± 0.4 |
| 2 | 5 | 317 ± 1 | 8.8 ± 0.1 | |
| 3 | 2.5 | Step 1: 2000 rpm 20 s Step 2: 5000 rpm 30 s | 138 ± 1 | 14.2 ± 0.4 |
| 4 | 5 | 349 ± 1 | 8.7 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczesny, R.; Scigala, A.; Derkowska-Zielinska, B.; Skowronski, L.; Cassagne, C.; Boudebs, G.; Viter, R.; Szłyk, E. Synthesis, Optical, and Morphological Studies of ZnO Powders and Thin Films Fabricated by Wet Chemical Methods. Materials 2020, 13, 2559. https://doi.org/10.3390/ma13112559
Szczesny R, Scigala A, Derkowska-Zielinska B, Skowronski L, Cassagne C, Boudebs G, Viter R, Szłyk E. Synthesis, Optical, and Morphological Studies of ZnO Powders and Thin Films Fabricated by Wet Chemical Methods. Materials. 2020; 13(11):2559. https://doi.org/10.3390/ma13112559
Chicago/Turabian StyleSzczesny, Robert, Aleksandra Scigala, Beata Derkowska-Zielinska, Lukasz Skowronski, Christophe Cassagne, Georges Boudebs, Roman Viter, and Edward Szłyk. 2020. "Synthesis, Optical, and Morphological Studies of ZnO Powders and Thin Films Fabricated by Wet Chemical Methods" Materials 13, no. 11: 2559. https://doi.org/10.3390/ma13112559
APA StyleSzczesny, R., Scigala, A., Derkowska-Zielinska, B., Skowronski, L., Cassagne, C., Boudebs, G., Viter, R., & Szłyk, E. (2020). Synthesis, Optical, and Morphological Studies of ZnO Powders and Thin Films Fabricated by Wet Chemical Methods. Materials, 13(11), 2559. https://doi.org/10.3390/ma13112559