3D Printed Model of Extrahepatic Biliary Ducts for Biliary Stent Testing
Abstract
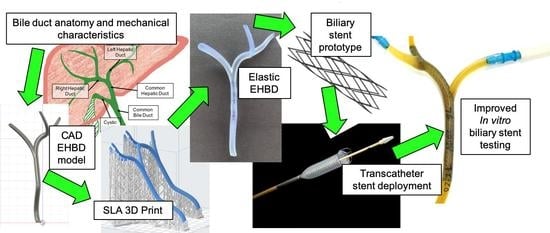
:1. Introduction
2. Materials and Methods
2.1. Computer Aided Design (CAD) and 3D Printing of the EHBD Model
2.2. Material Characterization of Elastic Polymers
2.3. EHBD Model Use in an In Vitro Biliary Stent Testing System
3. Results
3.1. CAD EHBD Model
3.2. 3D Printed EHBD Models
3.3. Mechanical Properties of Formlabs Elastic Polymer
3.4. Elastic EHBD Use in an In Vitro Biliary Stent Testing System
4. Discussion
4.1. Anatomic Accuracy of the FE EHBD Model
4.2. Tissue-Like Mechanical Properties of the 3D Printed FE EHBD Model
4.3. Comparison of In Vitro Biliary Stent Testing Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Gelrud, A.; Agarwal, B. Biliary strictures: Diagnostic considerations and approach. Gastroenterol. Rep. 2015, 3, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, B.K.; Karlsen, T.H.; Folseraas, T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1390–1400. [Google Scholar] [CrossRef]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Balls, M. Replacement of animal procedures: Alternatives in research, education and testing. Lab. Anim. 1994, 28, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Myers, D. From in vivo to in vitro: The medical device testing paradigm shift. ALTEX 2017, 479–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, K. Beyond the 3Rs: Expanding the use of human-relevant replacement methods in biomedical research. ALTEX 2019, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dua, K.S.; Reddy, N.D.; Rao, V.G.; Banerjee, R.; Medda, B.; Lang, I. Impact of reducing duodenobiliary reflux on biliary stent patency: An in vitro evaluation and a prospective randomized clinical trial that used a biliary stent with an antireflux valve. Gastrointest. Endosc. 2007, 65, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Bang, B.W.; Jeong, S.; Lee, D.H.; Lee, J.I.; Lee, S.C.; Kang, S.-G. The Biodurability of Covering Materials for Metallic Stents in a Bile Flow Phantom. Dig. Dis. Sci. 2012, 57, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.B.; Zhang, W.X.; Wan, X.J.; Yang, Q.; Qi, X.S.; Wang, X.P.; Lu, L.G. The effect of a novel drug-eluting plastic stent on biliary stone dissolution in an ex vivo bile perfusion model. Gastrointest. Endosc. 2014, 79, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cai, X.-B.; Guo, L.-L.; Qi, X.-S.; Gao, Q.; Wan, X.-J. Drug-eluting fully covered self-expanding metal stent for dissolution of bile duct stones in vitro. WJG 2019, 25, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Duch, B.U.; Andersen, H.; Gregersen, H. Mechanical Properties of the Porcine Bile Duct Wall. Biomed. Eng. Online 2004, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.C.; Zhang, H.M.; Li, J.; Dong, R.K.; Yao, B.C.; He, X.J.; Wang, H.Q.; Song, J. Comparison of Biomechanical Properties of Bile Duct between Pigs and Humans for Liver Xenotransplant. Transplant. Proc. 2013, 45, 741–747. [Google Scholar] [CrossRef]
- Yan, M.; Lewis, P.L.; Shah, R.N. Tailoring nanostructure and bioactivity of 3D-printable hydrogels with self-assemble peptides amphiphile (PA) for promoting bile duct formation. Biofabrication 2018, 10, 035010. [Google Scholar] [CrossRef] [PubMed]
- Tysoe, O.C.; Justin, A.W.; Brevini, T.; Chen, S.E.; Mahbubani, K.T.; Frank, A.K.; Zedira, H.; Melum, E.; Saeb-Parsy, K.; Markaki, A.E.; et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 2019, 14, 1884–1925. [Google Scholar] [CrossRef]
- Lewis, P.L.; Yan, M.; Su, J.; Shah, R.N. Directing the growth and alignment of biliary epithelium within extracellular matrix hydrogels. Acta Biomater. 2019, 85, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Brevini, T.; Tysoe, O.C.; Sampaziotis, F. Tissue engineering of the biliary tract and modelling of cholestatic disorders. J. Hepatol. 2020, S0168827820303676. [Google Scholar] [CrossRef]
- Lourenço, L.; Horta, D.; Rodrigues, C.; Canena, J.; Reis, J. Pseudocholangiocarcinoma Sign: Management of Portal Cavernoma Biliopathy with Fully-Covered Self-Expandable Metal Stent. Clin. Endosc. 2016, 50, 305–307. [Google Scholar] [CrossRef] [Green Version]
- Schulte, S.J.; Baron, R.L.; Teefey, S.A.; Rohrmann, C.A.; Freeny, P.C.; Shuman, W.P.; Foster, M.A. CT of the extrahepatic bile ducts: Wall thickness and contrast enhancement in normal and abnormal ducts. Am. J. Roentgenol. 1990, 154, 79–85. [Google Scholar] [CrossRef]
- Vakili, K.; Pomfret, E.A. Biliary Anatomy and Embryology. Surg. Clin. N. Am. 2008, 88, 1159–1174. [Google Scholar] [CrossRef]
- Tirkes, T.; Akisik, F. Gallbladder and Biliary Tree Anatomy, Variants, Cystic Lesions. In Abdominal Imaging; Hamm, B., Ros, P.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1241–1252. ISBN 978-3-642-13327-5. [Google Scholar]
- Boyer, J.L. Bile Formation and Secretion. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; p. c120027. ISBN 978-0-470-65071-4. [Google Scholar]
- Yoshida, J.; Chijiiwa, K.; Yamaguchi, K.; Yokohata, K.; Tanaka, M. Practical classification of the branching types of the biliary tree: An analysis of 1094 consecutive direct cholangiograms. J. Am. Coll. Surg. 1996, 182, 37–40. [Google Scholar]
- Mortelé, K.J.; Ros, P.R. Anatomic Variants of the Biliary Tree: MR Cholangiographic Findings and Clinical Applications. Am. J. Roentgenol. 2001, 177, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Peri, E.; Cescon, M.; Zanello, M.; Ercolani, G.; Zanfi, C.; Bertuzzo, V.; Di Gioia, P.; Pinna, A.D. Anatomic Variations of Intrahepatic Bile Ducts in a European Series and Meta-analysis of the Literature. J. Gastrointest. Surg. 2011, 15, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Gómez Zuleta, M.A.; Ruíz Morales, O.F.; Otero Rengino, W.A. ¿Cuál es el tamaño normal del conducto biliar común? Rev. Colomb. Gastroenterol. 2017, 32, 99. [Google Scholar] [CrossRef] [Green Version]
- Ramesh Babu, C.S.; Sharma, M. Biliary Tract Anatomy and its Relationship with Venous Drainage. J. Clin. Exp. Hepatol. 2014, 4, S18–S26. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.A.; Fulcher, A.S. The Cystic Duct: Normal Anatomy and Disease Processes. Radiographics 2001, 21, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Igami, T.; Ebata, T.; Yokoyama, Y.; Sugawara, G.; Mizuno, T.; Mori, K.; Ando, M.; Nagino, M. Surgical and Radiological Studies on the Length of the Hepatic Ducts. World J. Surg. 2015, 39, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, W.; Bird, N.; Chin, S.B.; Hill, N.; Johnson, A.G. On the mechanical behavior of the human biliary system. World J. Gastroenterol. 2007, 13, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Jian, C.; Wang, G. Biomechanical Study of the Bile Duct System outside the Liver. Bio-Med Mater. Eng. 1991, 1, 105–113. [Google Scholar] [CrossRef]
- Egorov, V.I.; Schastlivtsev, I.V.; Prut, E.V.; Baranov, A.O.; Turusov, R.A. Mechanical properties of the human gastrointestinal tract. J. Biomech. 2002, 35, 1417–1425. [Google Scholar] [CrossRef]
- Ladd, M.R.; Costello, C.M.; Gosztyla, C.; Werts, A.D.; Johnson, B.; Fulton, W.B.; Martin, L.Y.; Redfield, E.J.; Crawford, B.; Panaparambil, R.; et al. Development of Intestinal Scaffolds that Mimic Native Mammalian Intestinal Tissue. Tissue Eng. Part. A 2019, 25, 1225–1241. [Google Scholar] [CrossRef]
- Kwon, C.-I.; Kim, G.; Jeong, S.; Lee, W.S.; Lee, D.H.; Ko, K.H.; Hong, S.P.; Hahm, K.B. Bile Flow Phantom Model and Animal Bile Duct Dilation Model for Evaluating Biliary Plastic Stents with Advanced Hydrophilic Coating. Gut Liver 2016, 10, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Deshmukh, A.; Ye, Z.; Jang, Y.-Y. Efficient and Controlled Generation of 2D and 3D Bile Duct Tissue from Human Pluripotent Stem Cell-Derived Spheroids. Stem Cell Rev. Rep. 2016, 12, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Sampaziotis, F.; Justin, A.W.; Tysoe, O.C.; Sawiak, S.; Godfrey, E.M.; Upponi, S.S.; Gieseck, R.L.; de Brito, M.C.; Berntsen, N.L.; Gómez-Vázquez, M.J.; et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017, 23, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, Y.; Xiang, Y.; Liu, H.; Guo, R. A novel 3D printing PCL/GelMA scaffold containing USPIO for MRI-guided bile duct repair. Biomed. Mater. 2020, 15, 045004. [Google Scholar] [CrossRef] [PubMed]
- Schaub, J.R.; Huppert, K.A.; Kurial, S.N.T.; Hsu, B.Y.; Cast, A.E.; Donnelly, B.; Karns, R.A.; Chen, F.; Rezvani, M.; Luu, H.Y.; et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 2018, 557, 247–251. [Google Scholar] [CrossRef]
- Struecker, B.; Hillebrandt, K.-H.; Raschzok, N.; Jöhrens, K.; Butter, A.; Tang, P.; Andreou, A.; Napierala, H.; Reutzel-Selke, A.; Denecke, T.; et al. Implantation of a Tissue-Engineered Neo-Bile Duct in Domestic Pigs. Eur. Surg. Res. 2015, 56, 61–75. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Roh, T.; Lin, Y.; Ling, S.; Zhao, S.; Lin, J.D.; Khalil, N.; Cairns, D.M.; Manousiouthakis, E.; et al. Multifunctional Bioreactor System for Human Intestine Tissues. ACS Biomater. Sci. Eng. 2018, 4, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Penkala, R.; Abrahimi, P. A Perfusion Bioreactor for Intestinal Tissue Engineering. J. Surg. Res. 2007, 142, 327–331. [Google Scholar] [CrossRef]
- Hunsberger, J.; Simon, C.; Zylberberg, C.; Ramamoorthy, P.; Tubon, T.; Bedi, R.; Gielen, K.; Hansen, C.; Fischer, L.; Johnson, J.; et al. Improving patient outcomes with regenerative medicine: How the Regenerative Medicine Manufacturing Society plans to move the needle forward in cell manufacturing, standards, 3D bioprinting, artificial intelligence-enabled automation, education, and training. Stem Cells Transl. Med. 2020, 9, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, A.; Atala, A. Tissue Engineering: Toward a New Era of Medicine. Annu. Rev. Med. 2017, 68, 29–40. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Harron, K.; Gilbert, R. Research: Increasing value, reducing waste. Lancet 2014, 383, 1124. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Greenland, S.; Hlatky, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Formlabs Materials Medical. Available online: https://formlabs.com/industries/medical/materials/#elastic-50a-resin (accessed on 1 July 2020).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, J.; Patel, S.; Troop, L.; Guru, R.; Faist, N.; Bellott, B.J.; Esterlen, B.A. 3D Printed Model of Extrahepatic Biliary Ducts for Biliary Stent Testing. Materials 2020, 13, 4788. https://doi.org/10.3390/ma13214788
Thomas J, Patel S, Troop L, Guru R, Faist N, Bellott BJ, Esterlen BA. 3D Printed Model of Extrahepatic Biliary Ducts for Biliary Stent Testing. Materials. 2020; 13(21):4788. https://doi.org/10.3390/ma13214788
Chicago/Turabian StyleThomas, Joanna, Sagar Patel, Leia Troop, Robyn Guru, Nicholas Faist, Brian J. Bellott, and Bethany A. Esterlen. 2020. "3D Printed Model of Extrahepatic Biliary Ducts for Biliary Stent Testing" Materials 13, no. 21: 4788. https://doi.org/10.3390/ma13214788
APA StyleThomas, J., Patel, S., Troop, L., Guru, R., Faist, N., Bellott, B. J., & Esterlen, B. A. (2020). 3D Printed Model of Extrahepatic Biliary Ducts for Biliary Stent Testing. Materials, 13(21), 4788. https://doi.org/10.3390/ma13214788