Substituent Effect on Porphyrin Film-Gas Interaction by Optical Waveguide: Spectrum Analysis and Molecular Dynamic Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Molecular Dynamic Simulations
2.3. Spectrum and Morphology Analysis
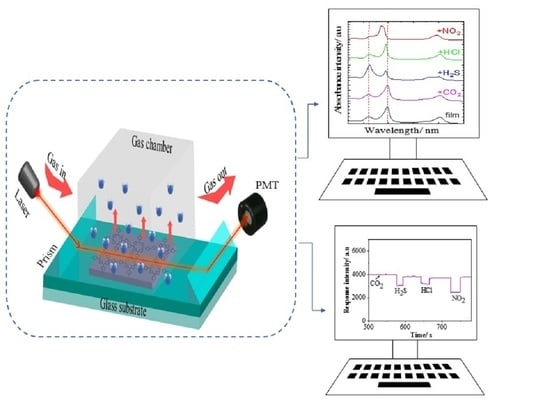
2.4. Optical Waveguide Detection
3. Results and Discussion
3.1. Molecular Dynamics Simulation Analysis
3.2. Spectrum Analysis
3.3. Gas Exposure
3.4. OWG Response Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yimit, A.; Rossberg, A.G.; Amemiya, T.; Itoh, K. Thin film composite optical waveguides for sensor applications: A review. Talanta 2005, 65, 1102–1109. [Google Scholar] [CrossRef]
- Fluitman, J.; Popma, T. Optical waveguide sensors. Sens. Actuators 1986, 10, 25–46. [Google Scholar] [CrossRef] [Green Version]
- Sarkisov, S.S.; Curley, M.J.; Boykin, C.; Diggs, D.E.; Grote, J.G.; Hopkins, F.K. Planar optical waveguide sensor of ammonia. In Proceedings of the Advanced Environmental, Chemical, and Biological Sensing Technologies II, Philadelphia, PA, USA, 7 December 2004; pp. 25–28. [Google Scholar]
- Abdukader, A.; Abliz, Y.; Mamtimin, M.; Kiminori, I. A planar optical waveguide sensor for hydrogen sulfide detection. Sens. Lett. 2007, 5, 395–397. [Google Scholar] [CrossRef]
- Maccraith, B.D.; Ruddy, V. Optical waveguide sensor using evanescent wave excitation of fluorescent dye in sol-gel glass. Electron. Lett. 1991, 27, 1247–1248. [Google Scholar] [CrossRef]
- Itoh, K.; Chen, X.M.; Murabayashi, M. Ion-exchanged glass optical waveguide systems for surface spectroscopy. composite structures for realization of high sensitivity and low loss. Chem. Lett. 1993, 22, 1991–1994. [Google Scholar] [CrossRef]
- Hamidi, S.M.; Bananej, A.; Tehranchi, M.M. Tunable optical properties in engineered one-dimensional coupled resonator optical waveguides. Opt. Laser Technol. 2012, 44, 1556–1563. [Google Scholar] [CrossRef]
- Zheltikov, A.M. The physical limit for the waveguide enhancement of nonlinear-optical processes. Opt. Spectrosc. 2003, 95, 410–415. [Google Scholar] [CrossRef]
- Mario, C.; Guo, Y.Y.; Michael, H. Porphyrins and phthalocyanines as materials for optical limiting. Synth. Met. 2004, 3, 231–243. [Google Scholar] [CrossRef]
- Purrello, R.; Gurrieri, S.; Lauceri, R. Porphyrin assemblies as chemical sensors. Coord. Chem. Rev. 1999, 190, 683–706. [Google Scholar] [CrossRef]
- Capan, İ.; Erdoğan, M.; Stanciu, G.A.; Stanciu, S.G.; Hristu, R.; Göktepe, M. The interaction between the gas sensing and surface morphology properties of lb thin films of porphyrins in terms of the adsorption kinetics. Mater. Chem. Phys. 2012, 136, 1130–1136. [Google Scholar] [CrossRef]
- Ogoshi, H.; Mizutani, T. Novel approaches to molecular recognition using porphyrins. Curr. Opin. Chem. Biol. 1999, 3, 736–739. [Google Scholar] [CrossRef]
- Cherian, S.; Wamser, C.C. Adsorption and Photoactivity of Tetra (4-carboxyphenyl) porphyrin (TCPP) on Nanoparticulate TiO2. J. Phys. Chem. B 2000, 104, 3624–3629. [Google Scholar] [CrossRef]
- Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R.; Di Natale, C.; D’Amico, A. Porphyrin-Based Nanostructures for Sensing Applications. J. Sens. 2009, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.; Rivera, M.; Rivera, J.; Amelines-Sarria, O.; Wang, Y. Different Interaction Mechanisms of Evaporated Porphyrin Films Exposed to NO2. Adv. Mater. Phys. Chem. 2018, 8, 441–457. [Google Scholar] [CrossRef] [Green Version]
- Alibabaei, L.; Wang, M.K.; Giovannetti, R.; Teuscher, J.; Di Censo, D.; Moser, J.E.; Comte, P.; Pucciarelli, F.; Zakeeruddin, S.M.; Gratzel, M. Application of Cu (II) and Zn (II) coproporphyrins as sensitizers for thin film dye sensitized solar cells. Energy Environ. Sci. 2010, 3, 956–961. [Google Scholar] [CrossRef] [Green Version]
- Giovannetti, R.; Zannotti, M.; Alibabaei, L.; Ferraro, S. Equilibrium and Kinetic Aspects in the Sensitization of Monolayer Transparent TiO2 Thin Films with Porphyrin Dyes for DSSC Applications. Int. J. Photoenergy 2014. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [Green Version]
- Zannotti, M.; Giovannetti, R.; Minofar, B.; Řeha, D.; Plačková, L.; D’Amato, C.A.; Rommozzi, E.; Dudko, H.V.; Kari, N.; Minicucci, M. Aggregation and metal-complexation behaviour of THPP porphyrin in ethanol/water solutions as function of pH. Spectrochim. Acta A 2018, 193, 235–248. [Google Scholar] [CrossRef]
- Giovannetti, R.; Alibabaei, L.; Petetta, L. Aggregation behaviour of a tetracarboxylic porphyrin in aqueous solution. J. Photochem. Photobiol. A. 2010, 211, 108–114. [Google Scholar] [CrossRef]
- Zannotti, M.; Giovannetti, R.; D’Amato, C.A.; Rommozzi, E. Spectroscopic studies of porphyrin functionalized multiwalled carbon nanotubes and their interaction with TiO2 nanoparticles surface. Spectrochim. Acta A 2016, 153, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gulgina, M.; Kari, N.; Renagul, A.; Patima, N.; Abliz, Y. 5, 10, 15, 20-tetrakis-(4-methoxyphenyl) porphyrin film/K+ ion-exchanged optical waveguide gas sensor. Opt. Laser Technol. 2020, 128. [Google Scholar] [CrossRef]
- Qingrong, M.; Kari, N.; Rena, S.; Yuan, Z.; Shawket, A.; Abliz, Y. Photoelectric conversion and protonation in sensor detection of SO2 and H2S using graphene oxide–tetrakis(4-carboxyphenyl) porphyrin. J. Appl. Remote Sens. 2020, 14. [Google Scholar] [CrossRef]
- Maimaiti, A.; Abdurahman, R.; Kari, N.; Ma, Q.; Wumaier, K.; Nizamidin, P.; Abliz, S.; Yimit, A. Highly Sensitive Optical Waveguide Sensor for SO2 and H2S Detection in the partspertrillion regime using Tetraaminophenyl Porphyrin. J. Mod. Opt. 2020, 67, 507–514. [Google Scholar] [CrossRef]
- Tuerdi, G.; Nizamidin, P.; Kari, N.; Yimit, A.; Fu, Y. Optochemical properties of gas-phase protonated tetraphenylporphyrin investigated using an optical waveguide NH3 sensor. RSC Adv. 2018, 8, 5614–5621. [Google Scholar] [CrossRef] [Green Version]
- Abudukeremu, H.; Kari, N.; Zhang, Y.; Wang, J.; Nizamidin, P.; Abliz, S.; Yimit, A. Highly sensitive free-base-porphyrin-based thin-film optical waveguide sensor for detection of low concentration NO2 gas at ambient temperature. J. Mater. Sci. 2018, 53, 10822–10834. [Google Scholar] [CrossRef]
- Buayishamu, K.; Kari, N.; Yuan, Z.; Patima, N.; Abliz, Y. Tetrahydroxyphenyl porphyrin membrane: A high-sensitivity optical waveguide gas sensor for NO2 detection. Meas. Sci. Technol. 2020, 31. [Google Scholar] [CrossRef]
- Çapan, İ.; Erdoğan, M.; Güner, B.M.; İlhan, B.; Stanciu, S.G.; Hristu, R.; Stanciu, G.A. Gas Sensing Properties of Porphyrin Thin Films Influenced by Their Surface Morphologies. Sens. Lett. 2014, 12, 1218–1227. [Google Scholar] [CrossRef]
- Clavian, L.M.; Rajesh Kumar, P.C.; Anil Kumar, K.V.; Narayana Rao, D.; Shihab, N.K.; Sanjeev, G. Enhanced third order optical nonlinearity in ultrathin amorphous film of tetraphenyl-porphyrin in picosecond regime. Opt. Laser Technol. 2019, 119. [Google Scholar] [CrossRef]
- Filimonova, Y.; Chulovskaya, S.; Kuzmin, S.; Parfenyuk, V. Influence of deposition condition on semiconductor properties of polyporphyrin films. Perspekt. Mater. 2019, 5–12. [Google Scholar] [CrossRef]
- Hofkens, J.; Latterini, L.; Vanoppen, P.; Faes, H.; Jeuris, K.; De Feyter, S.; Kerimo, J.; Barbara, P.F.; De Schryver, F.C.; Rowan, A.E.; et al. Mesostructure of Evaporated Porphyrin Thin Films: Porphyrin Wheel Formation. J. Phys. Chem B 1997, 101, 10588–10598. [Google Scholar] [CrossRef] [Green Version]
- Kuzmin, S.M.; Chulovskaya, S.A.; Parfenyuk, V.I. Effect of substituent structure on formation and properties of poly-hydroxyphenyl porphyrin films obtained by superoxide-assisted method. Electrochim. Acta 2020, 342. [Google Scholar] [CrossRef]
- Wales, D.J.; Parker, R.M.; Gates, J.C.; Grossel, M.C.; Smith, P.G.R. An investigation into relative humidity measurement using an aluminosilicate sol–gel thin film as the active layer in an integrated optical bragg grating refractometer. Sens. Actuators B Chem. 2013, 188, 857–866. [Google Scholar] [CrossRef]
- Wales, D.J.; Parker, R.M.; Quainoo, P.; Cooper, P.A.; Gates, J.C.; Grossel, M.C.; Smith, P.G.R. An integrated optical Bragg grating refractometer for volatile organic compound detection. Sens. Actuators B Chem. 2016, 232, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Girschikofsky, M.; Rosenberger, M.; Belle, S.; Brutschy, M.; Waldvogel, S.R.; Hellmann, R. Highly sensitive detection of naphthalene in solvent vapor using a functionalized pbg refractive index sensor. Sensors 2012, 12, 2018–2025. [Google Scholar] [CrossRef] [Green Version]
- Girschikofsky, M.; Rosenberger, M.; Belle, S.; Brutschy, M.; Waldvogel, S.R.; Hellmann, R. Optical planar bragg grating sensor for real-time detection of benzene, toluene and xylene in solvent vapour. Sens. Actuat. B Chem. 2012, 171, 338–342. [Google Scholar] [CrossRef]
- Girschikofsky, M.; Rosenberger, M.; Belle, S.; Brutschy, M.; Waldvogel, S.R.; Hellmann, R. Allylated cyclodextrins as effective affinity materials in chemical sensing of volatile aromatic hydrocarbons using an optical planar bragg grating sensor. Anal. Chim. Acta 2013, 791, 51–59. [Google Scholar] [CrossRef]
- Girschikofsky, M.; Ryvlin, D.; Waldvogel, S.; Hellmann, R. Optical sensor for real-time detection of trichlorofluoromethane. Sensors 2019, 19, 632. [Google Scholar] [CrossRef] [Green Version]
- Bonnett, R.; Ioannou, S.; James, A.G.; Andrew, G.J.; Pitt, C.W.; Soe, M.M.Z. Synthesis and film-forming properties of metal complexes of octadecyl ethers of 5,10,15,20-tetrakis (4-hydroxyphenyl) porphyrin. J. Mater. Chem. 1993, 3. [Google Scholar] [CrossRef]
- Case, D.A.; Darden, T.A.; Cheatham, T.E.I.; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Merz, K.M.; Wang, B.; Pearlman, D.A.; et al. AMBER 8 User’s Manual; University of California: San Francisco, CA, USA, 2004. [Google Scholar]
- Ghahremanpour, M.M.; van Maaren, P.J.; van der Spoel, D. The Alexandria library, a quantum-chemical database of molecular properties for force field development. Sci. Data 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Ghahremanpour, M.M.; van Maaren, P.J.; Ditz, J.C.; Lindh, R.; Roland, L.; van der Spoel, D. Large-Scale Calculations of Gas Phase Thermochemistry: Enthalpy of Formation, Standard Entropy and Heat Capacity. J. Chem. Phys. 2016, 145. [Google Scholar] [CrossRef] [Green Version]
- Ghahremanpour, M.M.; van Maaren, P.J.; Caleman, C.; Hutchison, G.R.; van der Spoel, D. Polarizable Drude Model with s-type Gaussian or Slater Charge Density for General Molecular Mechanics Force Fields. J. Chem. Theory Comput. 2018, 14, 5553–5566. [Google Scholar] [CrossRef] [PubMed]
- Walz, M.-M.; Ghahremanpour, M.M.; van Maaren, P.J.; van der Spoel, D. Phase-Transferable Force Field for Alkali Halides. J. Chem. Theory Comput. 2018, 14, 5933–5948. [Google Scholar] [CrossRef] [PubMed]
- Frisch, B.M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perceptionin molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Martínez, L. Packing optimization for automated generation of complexsystem’s initial configurations for molecular dynamics and docking. J. Comput. Chem. 2003, 24, 819–825. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N∙log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef] [Green Version]
- Van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1719. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visualmolecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kaye and Laby Tables of Physical and Chemical Constants. Available online: http://www.jacobnie.com/physics/atm_img/kay.html (accessed on 21 November 2020).
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound (accessed on 21 November 2020).
- Hollingsworth, J.V.; Richard, A.J.; Vicente, M.G.H.; Russo, P.S. Characterization of the Self-Assembly of meso -Tetra (4-sulfonatophenyl) porphyrin (H 2 TPPS 4−) in Aqueous Solutions. Biomacromolecules 2012, 13, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Tsuzuki, K.; Segawa, H. Homogeneously mixed porphyrin J-aggregates with rod-shaped nanostructures via zwitterionic self-assembly. Phys. Chem. Chem. Phys. 2011, 14, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, R. The Use of Spectrophotometry UV-Vis for the Study of Porphyrins. Macro Nano Spectrosc. 2012, 1, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Mcnaughton, A.J.; Richardson, T.H.; Barford, W.; Barford, W.; Dunbar, A.; Hutchinson, J.; Hunter, C.A. Characterisation of the reaction of free-base porphyrin to nitrogen dioxide. Colloids Surf. A Physicochem. Eng. Asp. 2006, 284, 345–349. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.; Guillén, M.G.; Lopes-Costa, T.; Castillero, P.; Barranco, A.; Gonzàlez-Elipe, A.R. Free-Base Carboxyphenyl Porphyrin Films Using a TiO2 Columnar Matrix: Characterization and Application as NO2 Sensors. Sensors 2015, 15, 11118–11132. [Google Scholar] [CrossRef] [Green Version]
- Soury, R.; Chaabene, M.; Jabli, M.; Saleh, T.A.; Been Chaabane, R.; Saint-Aman, E.; Loiseau, F.; Philouze, C.; Allouche, A.-R.; Nasri, H. Meso-tetrakis (3,4,5-trimethoxyphenyl) porphyrin derivatives: Synthesis, spectroscopic characterizations and adsorption of NO2. Chem. Eng. J. 2019, 375. [Google Scholar] [CrossRef]
- Ezhov, A.V.; Aleksandrov, A.E.; Zhdanova, K.A.; Zhdanov, A.P.; Klyukin, I.N.; Zhizhin, K.Y.; Mironov, A.F.; Tameev, A.R. Synthesis of Zn (II) porphyrin dyes and revealing an influence of their alkyl substituents on performance of dye-sensitized solar cells. Synth. Met. 2020, 269. [Google Scholar] [CrossRef]
- James, H. Lab Manual for Zumdahl/Zumdahl’s Chemistry, 6th ed.; Brooks Cole: Pacific Grove, CA, USA, 2002. [Google Scholar]
- Matsunaga, N.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Formulation of gas diffusion dynamics for thin film semiconductor gas sensor based on simple reaction-diffusion equation. Sens. Actuators B 2003, 96, 226–233. [Google Scholar] [CrossRef]
- Giovanna, D.L.; Andrea, R.; Luigi, M. Counteranion Dependent Protonation and Aggregation of Tetrakis (4-sulfonatophenyl) porphyrin in Organic Solvents. J. Phys. Chem. B 2006, 110, 7309–7315. [Google Scholar] [CrossRef]










| Porphyrins | Refractive Index | Gas Analytes | Refractive Index |
|---|---|---|---|
| TPP | 1.697 | Air | 1.000292 |
| TAPP | 1.762 | H2S | 1.000634 |
| TBPP | 1.610 | HCl | 1.000447 |
| TCPP | 1.744 | NO2 | 1.000297 |
| THPP | 1.746 | CO2 | 1.000449 |
| TMPP | 1.657 | NH3 | 1.000376 |
| TSPP | 1.724 | H2O (vapor) | 1.000256 |
| Porphyrins | Solvent | Molar Extinction Coefficient /ε (cm−1·mol−1·L) | ||||
|---|---|---|---|---|---|---|
| Soret Band (1 × 105) | Q4 (1 × 105) | Q3 (1 × 105) | Q2 (1 × 104) | Q1 (1 × 104) | ||
| TPP | CH2Cl2 | 417 nm 5.69 | 514 nm 1.95 | 548 nm 8.03 | 589 nm 5.32 | 645 nm 2.91 |
| TAPP | THF | 431 nm 1.87 | 523 nm 0.13 | 566 nm 0.97 | - | 661 nm 6.03 |
| TBPP | CH2Cl2 | 421 nm 2.89 | 518 nm 1.16 | 553 nm 0.89 | 592 nm 4.34 | 650 nm 2.87 |
| TCPP | CH3OH | 416 nm 6.32 | 513 nm 3.52 | 548 nm 2.02 | 589 nm 1.42 | 645 nm 9.66 |
| THPP | CH3OH | 418 nm 6.16 | 517 nm 1.90 | 554 nm 1.49 | 594 nm 6.16 | 649 nm 7.91 |
| TMPP | CH2Cl2 | 421 nm 2.90 | 518 nm 0.118 | 553 nm 0.90 | 592 nm 4.26 | 649 nm 2.87 |
| TSPP | DMF | 419 nm 7.19 | 515 nm 2.53 | 550 nm 1.35 | 591 nm 7.44 | 647 nm 6.84 |
| Porphyrins | Soret Band Before Gas Exposure (nm) | Soret Band After Gas Exposure (nm) | |||
|---|---|---|---|---|---|
| No Gas | CO2 | H2S | HCl | NO2 | |
| TPP | 437 | 462 | 455 | 456 | 497 |
| TAPP | 443 | 443 | 418 | 418 | 468 |
| TBPP | 434 | 460 | 461 | 457 | 455 |
| TCPP | 419 | 419 | 431 | 419 | 439 |
| THPP | 435 | 468 | 468 | 463 | 451 |
| TMPP | 448 | 448 | 476 | 475 | 470 |
| TSPP | 496 | 496 | 489 | 489 | 473 |
| Film | Absorbance Change (ΔA) at 670 nm After Gas Exposure | |||
|---|---|---|---|---|
| CO2 | H2S | HCl | NO2 | |
| TPP | 0.1380 | 0.1530 | 0.1730 | 0.0410 |
| TAPP | 0.0036 | 0.0120 | 0.0116 | 0.0072 |
| TBPP | 0.1038 | 0.1045 | 0.0772 | 0.1130 |
| TCPP | 0.0001 | 0.0006 | 0.0119 | 0.0419 |
| THPP | 0.0078 | 0.0078 | 0.0104 | 0.0500 |
| TMPP | 0.0050 | −0.0025 | −0.0076 | −0.0116 |
| TSPP | −0.0021 | 0.0230 | 0.0277 | −0.0436 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kari, N.; Zannotti, M.; Mamtmin, G.; Giovannetti, R.; Minofar, B.; Řeha, D.; Maimaiti, P.; Kutilike, B.; Yimit, A. Substituent Effect on Porphyrin Film-Gas Interaction by Optical Waveguide: Spectrum Analysis and Molecular Dynamic Simulation. Materials 2020, 13, 5613. https://doi.org/10.3390/ma13245613
Kari N, Zannotti M, Mamtmin G, Giovannetti R, Minofar B, Řeha D, Maimaiti P, Kutilike B, Yimit A. Substituent Effect on Porphyrin Film-Gas Interaction by Optical Waveguide: Spectrum Analysis and Molecular Dynamic Simulation. Materials. 2020; 13(24):5613. https://doi.org/10.3390/ma13245613
Chicago/Turabian StyleKari, Nuerguli, Marco Zannotti, Gulgina Mamtmin, Rita Giovannetti, Babak Minofar, David Řeha, Patigu Maimaiti, Buayishamu Kutilike, and Abliz Yimit. 2020. "Substituent Effect on Porphyrin Film-Gas Interaction by Optical Waveguide: Spectrum Analysis and Molecular Dynamic Simulation" Materials 13, no. 24: 5613. https://doi.org/10.3390/ma13245613
APA StyleKari, N., Zannotti, M., Mamtmin, G., Giovannetti, R., Minofar, B., Řeha, D., Maimaiti, P., Kutilike, B., & Yimit, A. (2020). Substituent Effect on Porphyrin Film-Gas Interaction by Optical Waveguide: Spectrum Analysis and Molecular Dynamic Simulation. Materials, 13(24), 5613. https://doi.org/10.3390/ma13245613