Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
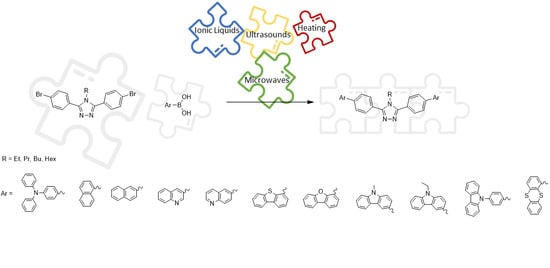
2.2. Synthesis of Compounds
2.2.1. The Synthesis of Suzuki Cross-Coupling Precursors
2.2.2. A General Procedure for Conventional Suzuki Cross-Coupling Reactions
2.2.3. A General Procedure for IL Alternative Approach for Suzuki Cross-Coupling Reactions
2.3. Characterization of Compounds
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kraft, A.; Grimsdale, A.C.; Holmes, A.B. Electroluminescent Conjugated Polymers—Seeing Polymers in a New Light. Angew. Chem. Int. Ed. 1998, 37, 402–428. [Google Scholar] [CrossRef]
- Segura, J.L. The chemistry of electroluminescent organic materials. Acta Polym. 1998, 49, 319–344. [Google Scholar] [CrossRef]
- Mitschke, U.; Bäuerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Bera, M.K.; Pal, P.; Malik, S. Solid-state emissive organic chromophores: Design, strategy and building blocks. J. Mater. Chem. C 2020, 8, 788–802. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, C. Blue fluorescent emitters: Design tactics and applications in organic light-emitting diodes. Chem. Soc. Rev. 2013, 42, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Ramsden, C.A.; Joule, J.A.; Zhdankin, V.V. Structure of Five-membered Rings with Two or More Heteroatoms. In Handbook of Heterocyclic Chemistry; Elsevier: Oxford, UK, 2010; Volume 2, pp. 139–209. [Google Scholar]
- Curtis, A.D.M.; Jennings, N. 1,2,4-Triazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, pp. 159–209. [Google Scholar]
- Liu, H.; Wang, L.; Wu, Y.; Liao, Q. Luminescence emission-modulated based on specific two-photon compound of triazole-conjugated pyrene derivative. RSC Adv. 2017, 7, 19002–19006. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yan, L.; Zhou, H.; You, W. A General Approach toward Electron Deficient Triazole Units to Construct Conjugated Polymers for Solar Cells. Chem. Mater. 2015, 27, 6470–6476. [Google Scholar] [CrossRef]
- Wu, C.-S.; Lee, S.-L.; Chen, Y. Bipolar copoly(aryl ether) containing distyrylbenzene, triphenylamine, and 1,2,4-triazole moieties: Synthesis and optoelectronic properties. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3099–3108. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Q.; Ao, L.; Zhong, C.; Yang, C.; Qin, J.; Ma, D. Highly Efficient Phosphorescent Organic Light-Emitting Diodes Hosted by 1,2,4-Triazole-Cored Triphenylamine Derivatives: Relationship between Structure and Optoelectronic Properties. J. Phys. Chem. C 2010, 114, 601–609. [Google Scholar] [CrossRef]
- Meng, X.; Chen, M.; Bai, R.; He, L. Cationic Iridium Complexes with 3,4,5-Triphenyl-4 H-1,2,4-Triazole Type Cyclometalating Ligands: Synthesis, Characterizations, and Their Use in Light-Emitting Electrochemical Cells. Inorg. Chem. 2020, 59, 9605–9617. [Google Scholar] [CrossRef]
- Demirbaş, Ü.; Özçifçi, Z.; Akçay, H.T.; Menteşe, E. Novel phthalocyanines bearing 1,2,4 triazole substituents: Synthesis, characterization, photophysical and photochemical properties. Polyhedron 2020, 181, 114470. [Google Scholar] [CrossRef]
- Abdurahman, A.; Wang, L.; Zhang, Z.; Feng, Y.; Zhao, Y.; Zhang, M. Novel triazole-based AIE materials: Dual-functional, highly sensitive and selective fluorescence probe. Dye. Pigment. 2020, 174, 108050. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, X.; Zhang, L.; Zhou, H.; Wu, L.; Cai, L. Electrochemical and quantum-chemical study on newly synthesized triazoles as corrosion inhibitors of mild steel in 1 M HCl. Res. Chem. Intermed. 2017, 43, 3145–3162. [Google Scholar] [CrossRef]
- Jiang, L.; Lan, Y.; He, Y.; Li, Y.; Li, Y.; Luo, J. 1,2,4-Triazole as a corrosion inhibitor in copper chemical mechanical polishing. Thin Solid Films 2014, 556, 395–404. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. Effects of 3-amino-1,2,4-triazole on the inhibition of copper corrosion in acidic chloride solutions. J. Colloid Interface Sci. 2007, 311, 144–151. [Google Scholar] [CrossRef]
- Wang, L. Inhibition of mild steel corrosion in phosphoric acid solution by triazole derivatives. Corros. Sci. 2006, 48, 608–616. [Google Scholar] [CrossRef]
- Shet, N.; Nazareth, R.; Suchetan, P.A. Corrosion inhibition of 316 stainless steel in 2M HCl by 4-{[4-(dimethylamino)benzylidene]amino}-5-methyl-4H-1,2,4-triazole-3-thiol. Chem. Data Collect. 2019, 20, 100209. [Google Scholar] [CrossRef]
- He, P.; Wu, B.; Shao, S.; Teng, T.; Wang, P.; Qu, X.-P. Characterization of 1,2,4-Triazole as Corrosion Inhibitor for Chemical Mechanical Polishing of Cobalt in H2O2 Based Acid Slurry. ECS J. Solid State Sci. Technol. 2019, 8, P3075–P3084. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Ba, Y.; Xu, Z. 1,2,4-Triazole-quinoline/quinolone hybrids as potential anti-bacterial agents. Eur. J. Med. Chem. 2019, 174, 1–8. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Kandeel, M.; Pillay, M.; Deb, P.K.; Abdallah, H.H.; Mahomoodally, M.F.; Chopra, D. Anti-Tubercular Properties of 4-Amino-5-(4-Fluoro-3-Phenoxyphenyl)-4H-1,2,4-Triazole-3-Thiol and Its Schiff Bases: Computational Input and Molecular Dynamics. Antibiotics 2020, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.C.; Patel, A.; Bhatt, H.G. New molecular insights into dual inhibitors of tankyrase as Wnt signaling antagonists: 3D-QSAR studies on 4H-1,2,4-triazole derivatives for the design of novel anticancer agents. Struct. Chem. 2020, 31, 2371–2389. [Google Scholar] [CrossRef]
- Saadaoui, I.; Krichen, F.; Ben Salah, B.; Ben Mansour, R.; Miled, N.; Bougatef, A.; Kossentini, M. Design, synthesis and biological evaluation of Schiff bases of 4-amino-1,2,4-triazole derivatives as potent angiotensin converting enzyme inhibitors and antioxidant activities. J. Mol. Struct. 2019, 1180, 344–354. [Google Scholar] [CrossRef]
- Fan, Y.-L.; Ke, X.; Liu, M. Coumarin-triazole Hybrids and Their Biological Activities. J. Heterocycl. Chem. 2018, 55, 791–802. [Google Scholar] [CrossRef]
- Kaur, P.; Chawla, A. 1,2,4-Triazole: A Review Of Pharmacological Activities. Parminder Kaur Anshul Chawla. Int. Res. J. Pharm 2017, 8, 10–29. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Gao, C.; Ren, Q.-C.; Chang, L.; Lv, Z.-S.; Feng, L.-S. Triazole derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 138, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Biological Potentials of Biological Active Triazole Derivatives: A Short Review. Org. Chem. Curr. Res. 2016, 5, 1000173. [Google Scholar] [CrossRef]
- Maddila, S.; Pagadala, R.; Jonnalagadda, S. 1,2,4-Triazoles: A Review of Synthetic Approaches and the Biological Activity. Lett. Org. Chem. 2013, 10, 693–714. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Wang, Y. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef]
- Sever, B.; Altıntop, M.D.; Demir, Y.; Pekdoğan, M.; Akalın Çiftçi, G.; Beydemir, Ş.; Özdemir, A. An extensive research on aldose reductase inhibitory effects of new 4H-1,2,4-triazole derivatives. J. Mol. Struct. 2020, 129446. [Google Scholar] [CrossRef]
- Hull, J.W.; Romer, D.R.; Adaway, T.J.; Podhorez, D.E. Development of Manufacturing Processes for a New Family of 2,6-Dihaloaryl 1,2,4-Triazole Insecticides. Org. Process Res. Dev. 2009, 13, 1125–1129. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, B.; He, Z.; Li, L.; Shi, H.; Wang, M. Enantioselective metabolism of four chiral triazole fungicides in rat liver microsomes. Chemosphere 2019, 224, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, G.-Y.; Zhang, Y.; Yang, M.-Y.; Ma, H.-X. Two Cu(II) complexes of 1,2,4-triazole fungicides with enhanced antifungal activities. Polyhedron 2019, 157, 163–169. [Google Scholar] [CrossRef]
- Verweij, P.E.; Kema, G.H.; Zwaan, B.; Melchers, W.J. Triazole fungicides and the selection of resistance to medical triazoles in the opportunistic mould Aspergillus fumigatus. Pest Manag. Sci. 2013, 69, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ribas e Ribas, A.D.; Spolti, P.; Del Ponte, E.M.; Donato, K.Z.; Schrekker, H.; Fuentefria, A.M. Is the emergence of fungal resistance to medical triazoles related to their use in the agroecosystems? A mini review. Braz. J. Microbiol. 2016, 47, 793–799. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, J.K.; Craig, I.R.; Brahm, L.; Fehr, M.; Weber, A.; Seet, M.; Mueller, B.; Wiebe, C.; Winter, C.H.; Grote, T.; et al. European Patent Application Substituted [1,2,4]triazoles as Agricultural Fungicides; European Patent Organisation: Dallas, TX, USA, 2020; pp. 1–79. [Google Scholar]
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef]
- Moulin, A.; Bibian, M.; Blayo, A.-L.; El Habnouni, S.; Martinez, J.; Fehrentz, J.-A. Synthesis of 3,4,5-Trisubstituted-1,2,4-triazoles. Chem. Rev. 2010, 110, 1809–1827. [Google Scholar] [CrossRef]
- Mustafa, S.; Nair, V.; Chittoor, J.; Krishnapillai, S. Synthesis of 1,2,4-Triazoles and Thiazoles from Thiosemicarbazide and its Derivatives. Mini Rev. Org. Chem. 2004, 1, 375–385. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M. Heterocyclization with Some Heterocyclic Diamines: Synthetic Approaches for Nitrogen Bridgehead Heterocyclic Systems. Heterocycles 2014, 89, 1125–1157. [Google Scholar] [CrossRef]
- Al-Masoudi, I.A.; Al-Soud, Y.A.; Al-Salihi, N.J.; Al-Masoudi, N.A. 1,2,4-Triazoles: Synthetic approaches and pharmacological importance. (Review). Chem. Heterocycl. Compd. 2006, 42, 1377–1403. [Google Scholar] [CrossRef]
- Jilloju, P.C.; Vinaykumar, A.; Shyam, P.; Vedula, R.R. One-pot, Multicomponent Cascade Reaction for the Synthesis of Various Aralkyl/alkylthio-3,5-dimethyl-1H-pyrazolyl-4H-1,2,4-triazol-4-amine and Their Docking Studies. J. Heterocycl. Chem. 2019, 56, 1012–1019. [Google Scholar] [CrossRef]
- Nikpassand, M.; Farshami, M.J. One-Pot Synthesis of Novel 3-Pyrazolyl-4H-1,2,4-triazoles Using Amino Glucose-Functionalized Silica-Coated NiFe2O4 Nanoparticles as a Magnetically Separable Catalyst. J. Clust. Sci. 2020, 1–8. [Google Scholar] [CrossRef]
- Jilloju, P.C.; Srikanth, M.; Kumar, S.V.; Vedula, R.R. One-Pot, Multi-Component Synthesis of Substituted 2-(6-Phenyl-7H-[1,2,4]Triazolo[3,4-b][1,3,4]Thiadiazin-3-yl)-2,3-Dihydrophthalazine-1,4-Diones. Polycycl. Aromat. Compd. 2020, 1–12. [Google Scholar] [CrossRef]
- de Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; de Meijere, A., Diederich, F., Eds.; Wiley-VCH: Weinheim, Germany, 2004; Volume 1. [Google Scholar]
- Miyaura, N. Metal-Catalyzed Cross-Coupling Reactions of Organoboron Compounds with Organic Halides. In Metal-Catalyzed Cross-Coupling Reactions; de Meijere, A., Diederich, F., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 41–123. [Google Scholar]
- Peruzzini, M.; Gonsalvi, L. Phosphorus Compounds. Advanced Tools in Catalysis and Material Sciences. In Catalysis by Metal Complexes; Springer: Berlin/Heidelberg, Germany, 2011; Volume 37. [Google Scholar]
- Mandal, B.; Ghosh, S.; Basu, B. Task-Specific Properties and Prospects of Ionic Liquids in Cross-Coupling Reactions. Top. Curr. Chem. 2019, 377, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Zahoor, A.F.; Akhtar, R.; Ahmad, M.; Naheed, S. Development of green methodologies for Heck, Chan–Lam, Stille and Suzuki cross-coupling reactions. Mol. Divers. 2019, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Boruah, P.R.; Gehlot, P.S.; Kumar, A.; Sarma, D. Palladium immobilized on the surface of MMT K 10 with the aid of [BMIM][BF4]: An efficient catalyst for Suzuki-Miyaura cross-coupling reactions. Mol. Catal. 2018, 461, 54–59. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent advances in the Suzuki-Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Lazzara, G.; Gruttadauria, M.; Milioto, S.; Noto, R. Green conditions for the Suzuki reaction using microwave irradiation and a new HNT-supported ionic liquid-like phase (HNT-SILLP) catalyst. Appl. Organomet. Chem. 2014, 28, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Kudelko, A.; Wróblowska, M.; Jarosz, T.; Katarzyna, Ł. Synthesis, spectral characteristics and electrochemistry of symmetrically substituted hybrids derived from 2,5-bis(4-bromophenyl)-1,3,4-oxadiazole under Suzuki cross-coupling reaction. Arkivoc 2015, 2015, 287–302. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Łapkowski, M. Efficient Synthesis of Conjugated 1,3,4-Thiadiazole Hybrids through Palladium-Catalyzed Cross-Coupling of 2,5-Bis(4-bromophenyl)-1,3,4-thiadiazole with Boronic Acids. Synlett 2015, 26, 2127–2130. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Kuźnik, N.; Łaba, K.; Łapkowski, M. Synthesis of Extended 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives in the Suzuki Cross-coupling Reactions. J. Heterocycl. Chem. 2017, 54, 1550–1557. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Synthesis of 4-Alkyl-4H-1,2,4-triazole Derivatives by Suzuki Cross-coupling Reactions and Their Luminescence Properties. Molecules 2019, 24, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, E. (Ed.) International Tables for Crystallography, Volume C: Mathematical, Physical and Chemical Tables; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: New York, NY, USA, 1991. [Google Scholar]
- Kruszynski, R.; Sierański, T. Can Stacking Interactions Exist beyond the Commonly Accepted Limits? Cryst. Growth Des. 2016, 16, 587–595. [Google Scholar] [CrossRef]
- Melhuish, W.H. Quantum efficiencies of fluorescence of organic substan ces: Effect of solvent and concentration of the fluorescent solute. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Birks, J.B.; Dyson, D.J. The relations between the fluorescence and absorption properties of organic molecules. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1963, 275, 135–148. [Google Scholar]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Shamsipur, M.; Chaichi, M.J. A study of quenching effect of sulfur-containing amino acids l-cysteine and l-methionine on peroxyoxalate chemiluminescence of 7-amino-4-trifluoromethylcumarin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1227–1231. [Google Scholar] [CrossRef]
- D’Auria, S.; Staiano, M.; Kuznetsova, I.M.; Turoverov, K.K. The Combined Use of Fluorescence Spectroscopy and X-Ray Crystallography Greatly Contributes to Elucidating Structure and Dynamics of Proteins. In Reviews in Fluorescence; Geddes, C.D., Lakowicz, J.R., Eds.; Springer: Boston, MA, USA, 2005; pp. 25–61. [Google Scholar]
- Kayumova, R.R.; Ostakhov, S.S.; Mamykin, A.V.; Muslukhov, R.R.; Iskhakova, G.F.; Ivanov, S.P.; Meshcheryakova, S.A.; Klen, E.E.; Khaliullin, F.A.; Kazakov, V.P. Structure and luminescence of thietane-containing 1,2,4-triazoles. Russ. J. Gen. Chem. 2011, 81, 1203–1210. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. The coordination chemistry of Zn(II), Cd(II) and Hg(II) complexes with 1,2,4-triazole derivatives. Dalton Trans. 2011, 40, 8475–8490. [Google Scholar] [CrossRef]
- Yunusova, S.N.; Novikov, A.S.; Khoroshilova, O.V.; Kolesnikov, I.E.; Demakova, M.Y.; Bolotin, D.S. Solid-state fluorescent 1,2,4-triazole zinc(II) complexes: Self-organization via bifurcated (N[sbnd]H)2⋯Cl contacts. Inorg. Chim. Acta 2020, 510, 119660. [Google Scholar] [CrossRef]
- Kędzia, A.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Microwave-promoted synthesis of highly luminescent s-tetrazine-1,3,4-oxadiazole and s-tetrazine-1,3,4-thiadiazole hybrids. Dye. Pigment. 2020, 172, 107865. [Google Scholar] [CrossRef]
- Meng, S.; Duan, A.; Xue, J.; Zheng, X.; Zhao, Y. UV-Vis, Fluorescence, and Resonance Raman Spectroscopic and Density Functional Theoretical Studies on 3-Amino-1,2,4-triazole: Microsolvation and Solvent-Dependent Nonadiabatic Excited State Decay in Solution. J. Phys. Chem. A 2018, 122, 8530–8538. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Braga, E.; Baluda, Y.; Kiskin, M.; Kryukova, M.; Karaush-Karmazin, N.; Baryshnikov, G.; Kuklin, A.; Minaev, B.; Ågren, H.; et al. Structure and tuneable luminescence in polymeric zinc compounds based on 3-(3-pyridyl)-5-(4-pyridyl)-1,2,4-triazole. Polyhedron 2020, 191, 114768. [Google Scholar] [CrossRef]
- Jin, X.D.; Li, B.; Gao, H.; Zhang, X.; Liu, W.Y. Synthesis, crystal structure, fluorescent property and DFT calculations of a new Zn(II) complex based on 3-(2-pyridyl)-5-(4-pyridyl)-1H-1,2,4-triazole. Chin. J. Struct. Chem. 2016, 35, 1129–1136. [Google Scholar] [CrossRef]
- Yao, Y.G.; Yang, J.X.; Zhang, X.; Qin, Y.Y. N-donor auxiliary ligand influence on the coordination mode variations of v-shaped triazole dicarboxylic acid ligand affording seven new luminescent Zn(II) compounds with variable structural motifs. Cryst. Growth Des. 2020, 20, 6366–6381. [Google Scholar] [CrossRef]







| Product | Yield a (%) | Stokes Shift b Δ (nm) | Quantum Yield c,d,e Φ | |||
|---|---|---|---|---|---|---|
| 7a | 97 | 351 | 352 | 443 | 92 | >0.98 c,d,e |
| 8a | 93 | 352 | 352 | 442 | 90 | >0.98 c,d,e |
| 9a | 99 | 351 | 351 | 443 | 92 | >0.98 c,d,e |
| 10a | 61 | 352 | 352 | 443 | 91 | >0.98 c,d,e |
 | |||||
| Entry | Substrate | Approach | Reaction Time | Additional Solvent (1 mL) | Yield a (%) |
|---|---|---|---|---|---|
| 1 | 5a | Conventional heating (oil bath 130 °C) | 24 h | - | - |
| 2 | Toluene | 85 | |||
| 3 | Ultrasounds | 10 h | - | 5 | |
| 4 | Toluene | 89 | |||
| 5 | Microwaves | 6 min | - | 8 | |
| 6 | Toluene | 94 | |||
| 7 | 5b | Conventional heating (oil bath 130 °C) | 24 h | - | - |
| 8 | Toluene | 80 | |||
| 9 | Ultrasounds | 10 h | - | 3 | |
| 10 | Toluene | 87 | |||
| 11 | Microwaves | 6 min | - | 10 | |
| 12 | Toluene | 89 | |||
| 13 | 5c | Conventional heating (oil bath 130 °C) | 24 h | - | - |
| 14 | Toluene | 85 | |||
| 15 | Ultrasounds | 10 h | - | 3 | |
| 16 | Toluene | 93 | |||
| 17 | Microwaves | 6 min | - | 9 | |
| 18 | Toluene | 97 | |||
| 19 | 5d | Conventional heating (oil bath 130 °C) | 24 vh | - | - |
| 20 | Toluene | 47 | |||
| 21 | Ultrasounds | 10 h | - | - | |
| 22 | Toluene | 56 | |||
| 23 | Microwaves | 6 min | - | 5 | |
| 24 | Toluene | 62 | |||
| Product | Yield a (%) | Stokes Shift b Δ (nm) | Quantum Yield c,d,e Φ | |||
|---|---|---|---|---|---|---|
| 7b | 88 | 297 | 304 | 382 | 85 | 0.75 c/0.74 d |
| 7c | 99 | 305 | 313 | 394 | 89 | >0.98 c,d,e |
| 7d | 79 | 305 | 312 | 395 | 90 | 0.75 c/0.74 d |
| 7e | 94 | 306 | 312 | 394 | 88 | 0.55 c/0.54 d |
| 7f | 96 | 290 | 291 | 371 | 81 | 0.11 c/0.11 d |
| 335 | 335 | 374 | 39 | 0.18 c/0.18 d | ||
| 7g | 92 | 286 | 290 | 388 | 102 | 0.63 c/0.62 d |
| 315 | 316 | 388 | 73 | 0.95 c/0.93 d | ||
| 7h | 70 | 300 | 304 | 403 | 103 | 0.77 c/0.76 d |
| 321 | 322 | 401 | 80 | >0.98 c,d,e | ||
| 7i | 76 | 301 | 304 | 404 | 103 | 0.78 c/0.77 d |
| 322 | 322 | 403 | 81 | >0.98 c,d,e | ||
| 7j | 77 | 293 | 293 | 409 | 116 | 0.77 c/0.76 d |
| 322 | 328 | 408 | 86 | >0.98 c,d,e | ||
| 7k | 87 | 263 | - | - | - | - |
| 9b | 62 | 296 | 304 | 382 | 86 | 0.64 c/0.62 d |
| 9c | 89 | 305 | 313 | 393 | 88 | 0.95 c/0.93 d |
| 9d | 99 | 304 | 311 | 395 | 91 | 0.40 c/0.39 d |
| 9e | 91 | 306 | 313 | 393 | 87 | 0.60 c/0.58 d |
| 9f | 93 | 290 | 291 | 373 | 83 | 0.14 c/0.14 d |
| 335 | 335 | 376 | 41 | 0.27 c/0.26 d | ||
| 9g | 88 | 286 | 292 | 387 | 101 | 0.76 c/0.75 d |
| 317 | 318 | 388 | 71 | >0.98 c,d,e | ||
| 9h | 97 | 300 | 304 | 402 | 102 | 0.80 c/0.78 d |
| 323 | 323 | 402 | 79 | >0.98 c,d,e | ||
| 9i | 98 | 301 | 304 | 403 | 102 | 0.78 c/0.77 d |
| 323 | 323 | 402 | 79 | >0.98 c,d,e | ||
| 9j | 91 | 293 | 295 | 409 | 116 | 0.81 c/0.79 d |
| 320 | 327 | 408 | 88 | >0.98 c,d,e | ||
| 9k | 86 | 263 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olesiejuk, M.; Kudelko, A.; Świątkowski, M. Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties. Materials 2020, 13, 5627. https://doi.org/10.3390/ma13245627
Olesiejuk M, Kudelko A, Świątkowski M. Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties. Materials. 2020; 13(24):5627. https://doi.org/10.3390/ma13245627
Chicago/Turabian StyleOlesiejuk, Monika, Agnieszka Kudelko, and Marcin Świątkowski. 2020. "Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties" Materials 13, no. 24: 5627. https://doi.org/10.3390/ma13245627
APA StyleOlesiejuk, M., Kudelko, A., & Świątkowski, M. (2020). Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties. Materials, 13(24), 5627. https://doi.org/10.3390/ma13245627