Phytosynthesized Metallic Nanoparticles—between Nanomedicine and Toxicology. A Brief Review of 2019′s Findings
Abstract
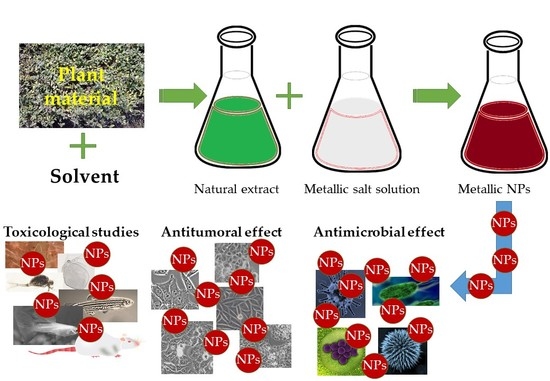
:1. Introduction
2. Antimicrobial Applications
3. Antitumoral Applications
4. Toxicological and Biocompatibility Studies
5. Recent Findings in the Morphology-Properties Correlation
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The toxicity of silver nanoparticles (AgNPs) to three freshwater invertebrates with different life strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Ortan, A.; Avramescu, S.M.; Fierascu, I. Phyto-nanocatalysts: Green synthesis, characterization and applications. Molecules 2019, 24, 3418. [Google Scholar] [CrossRef] [Green Version]
- Sana, S.S.; Dogiparthi, L.K. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mat. Lett. 2018, 226, 47–51. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Urbanowicz, R.A.; Thomson, B.J.; Irving, W.L.; Tarr, A.W.; Garnett, M.C. Enhanced nanoparticle uptake into virus infected cells: Could nanoparticles be useful in antiviral therapy? Int. J. Pharm. 2018, 547, 572–581. [Google Scholar] [CrossRef]
- Sutan, N.A.; Vilcoci, D.S.; Fierascu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fierascu, R.C. Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. rhizomes alcoholic extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular transport of silver and gold nanoparticles and biological responses: An update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Makhdoumi, P.; Taha, P.; Hossini, H.; Stelling, J.; Kamal, M.A.; Ashraf, G.M. A Review on Nano-Antimicrobials: Metal Nanoparticles, Methods and Mechanisms. Curr. Drug Metab 2017, 18, 120–128. [Google Scholar] [CrossRef]
- Brandelli, A.; Ritter, A.C.; Veras, F.F. Antimicrobial Activities of Metal Nanoparticles. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 337–363. [Google Scholar]
- Patil, M.P.; Kim, G.D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.L.; Jarrett, P.S. Antibacterial Silver. Met. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Hariram, M.; Vivekanandhan, S.; Ganesan, V.; Muthuramkumar, S.; Rodriguez-uribe, A.; Mohanty, A.K.; Misra, M. Tecoma stans flower extract assisted biogenic synthesis of functional Ag-Talc nanostructures for antimicrobial applications. Biores. Technol. Rep. 2019, 7, 100298. [Google Scholar] [CrossRef]
- Mtambo, S.E.; Krishna, S.B.N.; Govender, S.P. Physico-chemical, antimicrobial and anticancer properties of silver nanoparticles synthesised from organ-specific extracts of Bidens pilosa L. South Afr. J. Bot. 2019, 126, 196–206. [Google Scholar] [CrossRef]
- Ibrahim, E.H.; Kilany, M.; Ghramh, H.A.; Khan, K.A.; Ul Islam, S. Cellular proliferation/cytotoxicity and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J. Biol Sci. 2019, 26, 1689–1694. [Google Scholar] [CrossRef]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Varghese, R.; Almalki, M.A.; Ilavenil, S.; Rebecca, J.; Choi, K.C. Silver nanopaticles synthesized using the seed extract of Trigonella foenum-graecum L. and their antimicrobial mechanism and anticancer properties. Saudi J. Biol. Sci. 2019, 26, 148–154. [Google Scholar] [CrossRef]
- Hernández-Morales, L.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, L.E.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Núñez, G.; Espinoza, K.A. Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl Surf Sci 2019, 489, 952–961. [Google Scholar] [CrossRef]
- Dakshayani, S.S.; Marulasiddeshwara, M.B.; Sharath Kumar, M.N.; Ramesh, G.; Raghavendra Kumar, P.; Devaraja, S.; Hosamani, R. Antimicrobial, anticoagulant and antiplatelet activities of green synthesized silver nanoparticles using Selaginella (Sanjeevini) plant extract. Int. J. Biol. Macromol. 2019, 131, 787–797. [Google Scholar]
- Pontaza-Licona, Y.S.; Ramos-Jacques, A.L.; Cervantes-Chavez, J.A.; López-Miranda, J.L.; de Jesús Ruíz-Baltazar, A.; Maya-Cornejo, J.; Rodríguez-Morales, A.L.; Esparza, R.; Estevez, M.; Pérez, R.; et al. Alcoholic extracts from Paulownia tomentosa leaves for silver nanoparticles synthesis. Res. Phys. 2019, 12, 1670–1679. [Google Scholar] [CrossRef]
- Girón-Vázquez, N.G.; Gómez-Gutiérrez, C.M.; Soto-Robles, C.A.; Nava, O.; Lugo-Medina, E.; Castrejón-Sánchez, V.H.; Vilchis-Nestor, A.R.; Luque, P.A. Study of the effect of Persea americana seed in the green synthesis of silver nanoparticles and their antimicrobial properties. Res. Phys. 2019, 13, 102142. [Google Scholar] [CrossRef]
- Rashid, S.; Azeem, M.; Khan, S.A.; Shah, M.M.; Ahmad, R. Characterization and synergistic antibacterial potential of green synthesized silver nanoparticles using aqueous root extracts of important medicinal plants of Pakistan. Colloid. Surf. B Biointerfaces 2019, 179, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.K.M.; Kaviyarasu, K.; Ravindran, B.; Chang, S.W.; Arokiyaraj, S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B Biol. 2019, 191, 65–74. [Google Scholar] [CrossRef]
- Ashraf, A.; Zafar, S.; Zahid, K.; Salahuddin Shah, M.; Al-Ghanim, K.A.; Al-Misned, F.; Mahboob, S. Synthesis, characterization, and antibacterial potential of silver nanoparticles synthesized from Coriandrum sativum L. J. Infect. Public Health 2019, 12, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Malaikozhundan, B.; Saravanakumar, K.; Durán-Lara, E.F.; Wang, M.H.; Vaseeharan, B. Garlic clove extract assisted silver nanoparticle—Antibacterial, antibiofilm, antihelminthic, anti-inflammatory, anticancer and ecotoxicity assessment. J. Photochem. Photobiol. B Biol. 2019, 198, 111558. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Ravichandran, A.; Manoharan, V.; Muthukaruppan, R.; Somasundaram, S.; Pandi, B.; Krishnan, A.; Marimuthu, P.N.; Somasundaram, S.S.N.; You, S.G. Synthesis of Oldenlandia umbellata stabilized silver nanoparticles and their antioxidant effect, antibacterial activity, and bio-compatibility using human lung fibroblast cell line WI-38. Process. Biochem. 2019, 86, 196–204. [Google Scholar] [CrossRef]
- Al-Dhafri, K.; Ching, C.L. Phyto-synthesis of silver nanoparticles and its bioactivity response towards nosocomial bacterial pathogens. Biocatal. Agricult. Biotechnol. 2019, 18, 101075. [Google Scholar] [CrossRef]
- Majeed, S.; Bakhtiar, N.F.B.; Danish, M.; Ibrahim, M.N.M.; Hashim, R. Green approach for the biosynthesis of silver nanoparticles and its antibacterial and antitumor effect against osteoblast MG-63 and breast MCF-7 cancer cell lines. Sustain. Chem. Pharm. 2019, 12, 100138. [Google Scholar] [CrossRef]
- Rodríguez-Torres, M.P.; Acosta-Torres, L.S.; Díaz-Torres, L.A.; Padrón, G.H.; García-Contreras, R.; Millán-Chiu, B.E. Artemisia absinthium-based silver nanoparticles antifungal evaluation against three Candida species. Mat. Res. Expr. 2019, 6, 085408. [Google Scholar] [CrossRef]
- Hamid, H.A.; Mutazah, R. Synthesis of silver nanoparticles by Clinacanthus nutans extract supported with identification of flavonoids by UPLC-QTOF/MS and its antimicrobial activity. Iran J. Sci. Technol. Trans. Sci. 2019, 43, 2219–2225. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef] [Green Version]
- Alyousef, A.A.; Arshad, M.; Al Akeel, R.; Alqasim, A. Biogenic silver nanoparticles by Myrtus communis plant extract: Biosynthesis, characterization and antibacterial activity. Biotechnol. Biotechnol. Equip. 2019, 33, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Tanase, C.; Berta, L.; Coman, N.A.; Roșca, I.; Man, A.; Toma, F.; Mocan, A.; Jakab-Farkas, L.; Biró, D.; Mare, A. Investigation of in vitro antioxidant and antibacterial potential of silver nanoparticles obtained by biosynthesis using beech bark extract. Antioxidants 2019, 8, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayromlou, A.; Masoudi, S.; Mirzaie, A. Scorzonera calyculata aerial part extract mediated synthesis of silver nanoparticles: Evaluation of their antibacterial, antioxidant and anticancer activities. J. Clust. Sci. 2019, 30, 1037–1050. [Google Scholar] [CrossRef]
- Cyril, N.; George, J.B.; Joseph, L.; Raghavamenon, A.C.; Sylas, V.P. Assessment of antioxidant, antibacterial and anti-proliferative (lung cancer line A549) activities of green synthesized silver nanoparticles from Derris trifoliata. Toxicol. Res. 2019, 8, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haroon, M.; Zaidi, A.; Ahmed, B.; Rizvi, A.; Khan, M.S.; Musarrat, J. Effective inhibition of phytopathogenic microbes by eco-friendly leaf extract mediated silver nanoparticles (AgNPs). Indian J. Microbiol. 2019, 59, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of silver nanoparticles using aqueous extract of medicinal plants’ (Impatiens balsamina and Lantana camara) fresh land analysis of antimicrobial activity. Int. J. Microbiol. 2019, 2019, 8642303. [Google Scholar] [CrossRef] [Green Version]
- Jahan, I.; Erci, F.; Isildak, I. Microwave-assisted green synthesis of non-cytotoxic silver nanoparticles using the aqueous extract of Rosa santana (rose) petals and their antimicrobial activity. Anal. Lett. 2019, 52, 1860–1873. [Google Scholar] [CrossRef]
- Upadhyay, P.; Mishra, S.K.; Purohit, S.; Dubey, G.P.; Chauhan, B.S.; Srikrishna, S. Antioxidant, antimicrobial and cytotoxic potential of silver nanoparticles synthesized using flavonoid rich alcoholic leaves extract of Reinwardtia indica. Drug Chem. Toxicol 2019, 42, 65–75. [Google Scholar] [CrossRef]
- Razavi, R.; Molaei, R.; Moradi, M.; Tajik, H.; Ezati, P.; Yordshahi, A.S. Biosynthesis of metallic nanoparticles using mulberry fruit (Morus alba L.) extract for the preparation of antimicrobial nanocellulose film. Appl. Nanosci. 2019. [Google Scholar] [CrossRef]
- Labanni, A.; Zulhadjri, Z.; Handayani, D.; Ohya, Y.; Arief, S. The effect of monoethanolamine as stabilizing agent in Uncaria gambir Roxb. mediated synthesis of silver nanoparticles and its antibacterial activity. J. Disp. Sci. Technol. [CrossRef]
- De Matteis, V.; Rizzello, L.; Ingrosso, C.; Liatsi-Douvitsa, E.; De Giorgi, M.L.; De Matteis, G.; Rinaldi, R. Cultivar-dependent anticancer and antibacterial properties of silver nanoparticles synthesized using leaves of different Olea Europaea trees. Nanomaterials 2019, 9, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paosen, S.; Jindapol, S.; Soontarach, R.; Voravuthikunchai, S.P. Eucalyptus citriodora leaf extract-mediated biosynthesis of silver nanoparticles: Broad antimicrobial spectrum and mechanisms of action against hospital-acquired pathogens. APMIS 2019, 127, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, M.M.; Joshani, Z.; Zangeneh, A.; Miri, E. Green synthesis of silver nanoparticles using aqueous extract of Stachys lavandulifolia flower, and their cytotoxicity, antioxidant, antibacterial and cutaneous wound-healing properties. Appl. Organometal Chem. 2019, 33, 5016. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Ravi, S.O.A.S.; Ramachandran, A.; Ibrahim, I.A.A.; Nassir, A.M.; Yao, J. Synthesis of silver nanoparticles (AgNPs) from leaf extract of Salvia miltiorrhiza and its anticancer potential in human prostate cancer LNCaP cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2846–2854. [Google Scholar] [CrossRef] [Green Version]
- Krishnasamy Sekar, R.; Sridhar, A.; Perumalsamy, B.; Manikandan, D.B.; Ramasamy, T. In vitro antioxidant, antipathogenicity and cytotoxicity effect of silver nanoparticles fabricated by onion (Allium cepa L.) peel extract. BioNanoSci 2019. [Google Scholar] [CrossRef]
- Martinez de Tejada, G.; Sanchez-Gomez, S.; Razquin-Olazaran, I.; Kowalski, I.; Kaconis, Y.; Heinbockel, L.; Andra, J.; Schurholz, T.; Hornef, M.; Dupont, A.; et al. Bacterial cell wall compounds as promising targets of antimicrobial agents I. Antimicrobial peptides and lipopolyamines. Curr. Drug Targets 2012, 13, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, I.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Anuta, V.; Velescu, B.S.; Jinga, M.; Jinga, V. A short overview of recent developments on antimicrobial coatings based on phytosynthesized metal nanoparticles. Coatings 2019, 9, 787. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.A.; Eisa, N.E.; Virk, P.; Hendi, A.A.; Ortashi, K.M.O.O.; Mahgoub, A.A.S.A.; Elobeid, M.A.; Eissa, F.Z. Green synthesis of gold nanoparticles: Preparation, characterization, cytotoxicity, and anti-bacterial activities. Mat. Lett. 2019, 256, 126608. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Ali, D.M.; Loke, M.F.; Thajuddin, N.; Alharbi, N.S.; Yadavalli, T.; Alagiri, M.; Vadivelu, J. Anti-Helicobacter pylori, cytotoxicity and catalytic activity of biosynthesized gold nanoparticles: Multifaceted application. Arab. J. Chem. 2019, 12, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Zhaleh, M.; Zangeneh, A.; Goorani, S.; Seydi, N.; Zangeneh, M.M.; Tahvilian, R.; Pirabbasi, E. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl. Organometal. Chem. 2019, 33, 5015. [Google Scholar] [CrossRef]
- Sunderam, V.; Thiyagarajan, D.; Lawrence, A.V.; Mohammed, S.S.S.; Selvaraj, A. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi. J. Biol. Sci. 2019, 26, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Vinosha, M.; Palanisamy, S.; Muthukrishnan, R.; Selvam, S.; Kannapiran, E.; You, S.G.; Prabhu, N.M. Biogenic synthesis of gold nanoparticles from Halymenia dilatata for pharmaceutical applications: Antioxidant, anti-cancer and antibacterial activities. Proc. Biochem. 2019, 85, 219–229. [Google Scholar] [CrossRef]
- Tahvilian, R.; Zangeneh, M.M.; Falahi, H.; Sadrjavadi, K.; Jalalvand, A.R.; Zangeneh, A. Green synthesis and chemical characterization of copper nanoparticles using Allium saralicum leaves and assessment of their cytotoxicity, antioxidant, antimicrobial, and cutaneous wound healing properties. Appl. Organometal. Chem. 2019, 33, 5234. [Google Scholar] [CrossRef]
- Johnson, A.; Uwa, P. Eco-friendly synthesis of iron nanoparticles using Uvaria chamae: Characterization and biological activity. Inorg. Nano-Met. Chem. 2019, 49, 431–442. [Google Scholar] [CrossRef]
- Seydi, N.; Saneei, S.; Jalalvand, A.R.; Zangeneh, M.M.; Zangeneh, A.; Tahvilian, R.; Pirabbasi, E. Synthesis of titanium nanoparticles using Allium eriophyllum Boiss aqueous extract by green synthesis method and evaluation of their remedial properties. Appl. Organometal. Chem. 2019, 33, 5191. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef] [Green Version]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Shanavas, S.; Duraimurugan, J.; Kumar, G.S.; Ramesh, R.; Acevedo, R.; Anbarasan, P.M.; Maadeswaran, P. Ecofriendly green synthesis of ZnO nanostructures using Artabotrys Hexapetalu and Bambusa Vulgaris plant extract and investigation on their photocatalytic and antibacterial activity. Mater. Res. Express. 2019, 6, 105098. [Google Scholar] [CrossRef]
- Hussain, A.; Oves, M.; Alajmi, M.F.; Hussain, I.; Amir, S.; Ahmed, J.; Rehman, M.T.; El-Seedif, H.R.; Ali, I. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: Anticancer and antimicrobial activities. RSC Adv. 2019, 9, 15357–15369. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, H.; Nakara, A.; Menon, S.; Shanmugam, V.K. Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum Tamala leaf extract and its promising effect towards the antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101212. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ahmad, R.; Ashraf, M. Plant-extract mediated green approach for the synthesis of ZnONPs: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. J. Mol. Struct. 2019, 1189, 315–327. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Nagabhushana, H.; Nagaraju, G. Euphorbia heterophylla (L.) mediated fabrication of ZnO NPs: Characterization and evaluation of antibacterial and anticancer properties. Biocatal. Agricult. Biotechnol. 2019, 18, 100894. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Rajkumar, K.S.; Arun, S.; Babu, M.D.; Balaji, P.; Sivasubramanian, S.; Vignesh, V.; Thirumurugan, R. Facile biofabrication, characterization, evaluation of photocatalytic, antipathogenic activity and in vitro cytotoxicity of zinc oxide nanoparticles. Biocatal. Agricult. Biotechnol. 2019, 22, 101436. [Google Scholar] [CrossRef]
- Chemingui, H.; Missaoui, T.; Mzali, J.C.; Yildiz, T.; Konyar, M.; Smiri, M.; Saidi, N.; Hafiane, A.; Yatmaz, H.C. Facile green synthesis of zinc oxide nanoparticles (ZnO NPs): Antibacterial and photocatalytic activities. Mat. Res. Express 2019, 6, 1050b4. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Mahmood, T.; Qyyum, A.; Kanwal, S. Biofabrication of iron oxide nanoparticles by leaf extract of Rhamnus virgata: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. Appl. Organometal. Chem. 2019, 33, 4947. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N. Ultrasound assisted-phytofabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2405–2423. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Abdallah, Y.; Ogunyemi, S.O.; Abdelazez, A.; Zhang, M.; Hong, X.; Ibrahim, E.; Hossain, A.; Fouad, H.; Li, B.; Chen, J. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L. (rosemary) and the antibacterial activities against Xanthomonas oryzae pv. Oryzae. BioMed. Res. Int. 2019, 2019, 5620989. [Google Scholar] [CrossRef] [Green Version]
- Sabouri, Z.; Akbari, A.; Hosseini, H.A.; Hashemzadeh, A.; Darroudi, M. Eco-friendly biosynthesis of nickel oxide nanoparticles mediated by Okra plant extract and investigation of their photocatalytic, magnetic, cytotoxicity, and antibacterial properties. J. Clust. Sci. 2019, 30, 1425–1434. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Batool, R.; Khalil, A.T.; Hameed, S.; Kanwal, S.; Ullah, I.; Mahmood, T. Biogenic synthesis of green and cost effective cobalt oxide nanoparticles using Geranium wallichianum leaves extract and evaluation of in vitro antioxidant, antimicrobial, cytotoxic and enzyme inhibition properties. Mater. Res. Express 2019, 6, 115407. [Google Scholar] [CrossRef]
- Rao, T.N.; Babji, R.P.; Ahmad, N.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; Husain, F.M. Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi. J. Biol. Sci. 2019, 26, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Syed, B.; Karthik, N.; Bhat, P.; Bisht, N.; Prasad, A.; Satish, S.; Prasad, M.N.N. Phyto-biologic bimetallic nanoparticles bearing antibacterial activity against human pathogens. J. King Saud. Univ. Sci. 2019, 31, 798–803. [Google Scholar] [CrossRef]
- Lagashetty, A.; Ganiger, S.K.; Shashidhar. Synthesis, characterization and antibacterial study of Ag–Au Bi-metallic nanocomposite by bioreduction using Piper betle leaf extract. Heliyon 2019, 5, 02794. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, A.E.; Oke, A.M.; Lateef, A.; Oyatokun, A.A.; Abisoye, O.D.; Adiji, I.P.; Fagbenro, D.O.; Amusan, T.W.; Badmus, J.A.; Asafa, T.B.; et al. Biosynthesis of silver, gold and silver–gold alloy nanoparticles using Persea americana fruit peel aqueous extract for their biomedical properties. Nanotechnol. Environ. Eng. 2019, 4, 13. [Google Scholar] [CrossRef]
- Khan, I.; Behera, S.K.; Paul, P.; Das, B.; Suar, M.; Jayabalan, R.; Fawcett, D.; Poinern, G.E.J.; Tripathy, S.K.; Mishra, A. Biogenic Au@ZnO core–shell nanocomposites kill Staphylococcus aureus without provoking nuclear damage and cytotoxicity in mouse fibroblasts cells under hyperglycemic condition with enhanced wound healing proficiency. Med. Microbiol. Immunol. 2019, 208, 609–629. [Google Scholar] [CrossRef]
- Heydari, R.; Koudehi, M.F.; Pourmortazavi, S.M. Antibacterial activity of Fe3O4/Cu nanocomposite: Green synthesis using Carum carvi L. seeds aqueous extract. ChemistrySelect 2019, 4, 531–535. [Google Scholar] [CrossRef]
- Fierascu, I.; Georgiev, M.I.; Ortan, A.; Fierascu, R.C.; Avramescu, S.M.; Ionescu, D.; Sutan, A.; Brinzan, A.; Ditu, L.M. Phyto-mediated metallic nanoarchitectures via Melissa officinalis L.: Synthesis, characterization and biological properties. Sci. Rep. 2017, 7, 12428. [Google Scholar] [CrossRef] [Green Version]
- Akter, M.; Sikderb, T.; Rahman, M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Chavata, R.; Datchanamurthy, S.; Kotteeswaran, V. Biofabrication of silver nanoparticles from aqueous leaf extract of Leucas aspera and their anticancer activity on human cervical cancer cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 045008. [Google Scholar] [CrossRef]
- Masese, O.B.; Selvi, S. Cytotoxic effects of Ceiba pentandra L. mediated silver nanoparticles on HCT-116 colon cancer cell lines through ROS generation and cell membrane damage. Int. J. Res. Pharm. Sci. 2019, 10, 3236–3243. [Google Scholar]
- El-Hawary, S.S.; El-Hefnawy, H.M.; Osman, S.M.; Mostafa, E.S.; Mokhtar, F.A.; El-Raey, M.A. Chemical profile of two Jasminum sambac l. (Ait) cultivars cultivated in Egypt–their mediated silver nanoparticles synthesis and selective cytotoxicity. Int. J. Appl. Pharm. 2019, 11, 154–164. [Google Scholar] [CrossRef]
- Gajendran, B.; Durai, P.; Varier, K.M.; Liu, W.; Li, Y.; Rajendran, S.; Nagarathnam, R.; Chinnasamy, A. Green synthesis of silver nanoparticle from Datura inoxia flower extract and its cytotoxic activity. BioNanoScience 2019, 9, 564–572. [Google Scholar] [CrossRef]
- Mohammadi, G.; Zangeneh, M.M.; Zangeneh, A.; Haghighi, Z.M.S. Chemical characterization and anti-breast cancer effects of silver nanoparticles using Phoenix dactylifera seed ethanolic extract on 7,12-Dimethylbenz[a] anthracene-induced mammary gland carcinogenesis in Sprague Dawley male rats. Appl. Organometal. Chem. 2019, 5136. [Google Scholar] [CrossRef]
- Rohini, B.; Akther, T.; Waseem, M.; Khan, J.; Kashif, M.; Hemalatha, S. AgNPs from Nigella sativa control breast cancer: An in vitro study. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 185–194. [Google Scholar] [CrossRef]
- Yadav, R.; Saini, H.; Kumar, D.; Pasi, S.; Agrawal, V. Bioengineering of Piper longum L. extract mediated silver nanoparticles and their potential biomedical applications. Mat. Sci. Eng. C 2019, 104, 109984. [Google Scholar] [CrossRef]
- Shaniba, V.S.; Aziz, A.A.; Jayasree, P.R.; Kumar, P.R.M. Manilkara zapota (L.) P. Royen leaf extract derived silver nanoparticles induce apoptosis in human colorectal carcinoma cells without affecting human lymphocytes or erythrocytes. Biol. Trace Elem. Res. 2019, 192, 160–174. [Google Scholar] [CrossRef]
- Karuppaiya, P.; Satheeshkumar, E.; Tsay, H.S. Biogenic synthesis of silver nanoparticles using rhizome extract of Dysosma pleiantha and its antiproliferative effect against breast and human gastric cancer cells. Mol. Biol. Rep. 2019, 46, 4725–4734. [Google Scholar] [CrossRef]
- Odeyemi, S.W.; De La Mare, J.; Edkins, A.L.; Afolayan, A.J. In vitro and in vivo toxicity assessment of biologically synthesized silver nanoparticles from Elaeodendron croceum. J. Complement Integr. Med. 2019, 16, 0184. [Google Scholar] [CrossRef]
- Vinay, S.P.; Udayabhanu; Nagaraju, G.; Chandrappa, C.P.; Chandrasekhar, N. Rauvolfia tetraphylla (Devil pepper)-mediated green synthesis of Ag nanoparticles: Applications to anticancer, antioxidant and antimitotic. J. Clust. Sci. 2019, 30, 1545–1564. [Google Scholar] [CrossRef]
- Sutan, N.A.; Vilcoci, D.S.; Fierascu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fierascu, R.C. Influence of the phytosynthesis of noble metal nanoparticles on the cytotoxic and genotoxic effects of Aconitum toxicum Reichenb. leaves alcoholic extract. J. Clust. Sci. 2019, 30, 647–660. [Google Scholar] [CrossRef]
- Patil, M.P.; Bayara, E.; Subedi, P.; Piad, L.L.A.; Tarte, N.H.; Kim, G.D. Biogenic synthesis, characterization of gold nanoparticles using Lonicera japonica and their anticancer activity on HeLa cells. J. Drug. Deliv. Sci. Technol. 2019, 51, 83–90. [Google Scholar] [CrossRef]
- Sun, B.; Hu, N.; Han, L.; Pi, Y.; Gao, Y.; Chen, K. Anticancer activity of green synthesised gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4012–4019. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Ahn, E.Y.; Park, Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef]
- Majumdar, M.; Biswas, S.C.; Choudhury, R.; Upadhyay, P.; Adhikary, A.; Roy, D.N.; Misra, T.K. Synthesis of gold nanoparticles using Citrus macroptera fruit extract: Anti-biofilm and anticancer activity. ChemistrySelect 2019, 4, 5714–5723. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Li, F. Synthesis of gold nanoparticles from leaf Panax notoginseng and its anticancer activity in pancreatic cancer PANC-1 cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1216–1223. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Su, W.; Wang, Y.; Dang, M.; Zhang, W.; Wang, C. Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhu, J.; Li, G.; Wang, J.; Veeraraghavan, V.P.; Mohan, S.K.; Zhang, Q. Biologically synthesized green gold nanoparticles from Siberian ginseng induce growth-inhibitory effect on melanoma cells (B16). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3297–3305. [Google Scholar] [CrossRef] [Green Version]
- Virmani, I.; Sasi, C.; Priyadarshini, E.; Kumar, R.; Sharma, S.K.; Singh, G.P.; Pachwarya, R.B.; Paulraj, R.; Barabadi, H.; Saravanan, M.; et al. Comparative anticancer potential of biologically and chemically synthesized gold nanoparticles. J. Clust. Sci. 2019. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Z.; Jia, K.; Zhang, W.; Dang, M. Rabdosia rubescens Linn: Green synthesis of gold nanoparticles and their anticancer effects against human lung cancer cells A549. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2171–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Tiwari, R.; Singh, V.K.; Singh, P.; Khadim, S.R.; Singh, U.; Laxmi; Srivastava, V.; Hasan, S.H.; Asthana, R.K. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J. Drug Deliv. Sci. Technol. 2019, 51, 164–176. [Google Scholar] [CrossRef]
- Mohammad, G.R.K.S.; Tabrizi, M.H.; Ardalan, T.; Yadamani, S.; Safavi, E. Green synthesis of zinc oxide nanoparticles and evaluation of anti-angiogenesis, anti-inflammatory and cytotoxicity properties. J. Biosci. 2019, 44, 30. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Hosseini, H.A.; Hashemzadeh, A.; Mohajeri, M.; Sabouri, Z.; Darroudi, M.; Oskuee, R.K. Cytotoxicity and photocatalytic applications of biosynthesized ZnO nanoparticles by Rheum turketanicum rhizome extract. Mater. Res. Express 2019, 6, 125016. [Google Scholar] [CrossRef]
- Tettey, C.O.; Shin, H.M. Evaluation of the antioxidant and cytotoxic activities of zinc oxide nanoparticles synthesized using Scutellaria baicalensis root. Sci. Afr. 2019, 6, 00157. [Google Scholar] [CrossRef]
- Asik, R.M.; Gowdhami, B.; Jaabir, M.S.M.; Archunan, G.; Suganthy, N. Anticancer potential of zinc oxide nanoparticles against cervical carcinoma cells synthesized via biogenic route using aqueous extract of Gracilaria edulis. Mat. Sci. Eng. C 2019, 103, 109840. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.V.N.; Pallela, P.V.K.; Padavala, V.S.; Kolapalli, V.R.M. Antibiotic potentiation and anti-cancer competence through bio-mediated ZnO nanoparticles. Mat. Sci. Eng. C 2019, 103, 109756. [Google Scholar] [CrossRef]
- Shahnaz, M.; Danish, M.; Bin Ismail, M.H.; Ansari, M.T.; Ibrahim, M.N.M. Anticancer and apoptotic activity of biologically synthesized zinc oxide nanoparticles against human colon cancer HCT-116 cell line- in vitro study. Sustain. Chem. Pharm. 2019, 14, 100179. [Google Scholar]
- Rahmani, R.; Gharanfoli, M.; Gholamin, M.; Darroudi, M.; Chamani, J.; Sadrid, K. Green synthesis of 99mTc-labeled-Fe3O4 nanoparticles using Quince seeds extract and evaluation of their cytotoxicity and biodistribution in rats. J. Mol. Struct. 2019, 1196, 394–402. [Google Scholar] [CrossRef]
- Muhammad, W.; Khan, M.A.; Nazir, M.; Siddiquah, A.; Mushtaq, S.; Hashmi, S.S.; Abbasi, B.H. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mat. Sci. Eng. C 2019, 103, 109740. [Google Scholar] [CrossRef]
- Lourenço, I.M.; Pieretti, J.C.; Nascimento, M.H.M.; Lombello, C.B.; Seabra, A.B. Eco-friendly synthesis of iron nanoparticles by green tea extract and cytotoxicity effects on tumoral and non-tumoral cell lines. Energ. Ecol. Environ. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Nezhad, S.A.; Es-haghi, A.; Tabrizi, M.H. Green synthesis of cerium oxide nanoparticle using Origanum majorana L. leaf extract, its characterization and biological activities. Appl. Organomet Chem. 2019, 5314. [Google Scholar]
- Javadi, F.; Yazdi, M.E.T.; Baghani, M.; Es-haghi, A. Biosynthesis, characterization of cerium oxide nanoparticles using Ceratonia siliqua and evaluation of antioxidant and cytotoxicity activities. Mater. Res. Express 2019, 6, 065408. [Google Scholar] [CrossRef]
- Miri, A.; Darroudi, M.; Sarani, M. Biosynthesis of cerium oxide nanoparticles and its cytotoxicity survey against colon cancer cell line. Appl. Organomet Chem. 2019, 5308. [Google Scholar] [CrossRef]
- Krishnan, V.; Loganathan, C.; Thayumanavan, P. Green synthesized selenium nanoparticle as carrier and potent delivering agent of s-allyl glutathione: Anticancer effect against hepatocarcinoma cell line (HepG2) through induction of cell cycle arrest and apoptosis. J. Drug Deliv. Sci. Technol. 2019, 53, 101207. [Google Scholar] [CrossRef]
- Alijani, H.Q.; Pourseyedi, S.; Mahani, M.T.; Khatami, M. Green synthesis of zinc sulfide (ZnS) nanoparticles using Stevia rebaudiana Bertoni and evaluation of its cytotoxic properties. J. Mol. Struct. 2019, 1175, 214–218. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Singh, M. Eco-friendly synthesis of copper oxide, zinc oxide and copper oxide–zinc oxide nanocomposites, and their anticancer applications. J. Inorg. Organomet Polym. 2019. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Shameli, K.; Miyake, M.; Teow, S.Y.; Peh, S.C.; Mohamad, S.E.; Husna, S.; Taib, M. Green fabrication of biologically active magnetic core-shell Fe3O4/Au nanoparticles and their potential anticancer effect. Mat. Sci. Eng. C 2019, 96, 51–57. [Google Scholar] [CrossRef]
- Jenifer, A.A.; Malaikozhundan, B.; Vijayakumar, S.; Anjugam, M.; Iswarya, A.; Vaseeharan, B. Green synthesis and characterization of silver nanoparticles (AgNPs) using leaf extract of Solanum nigrum and assessment of toxicity in vertebrate and invertebrate aquatic animals. J. Clust. Sci. 2019. [Google Scholar] [CrossRef]
- Yun, R.; Li, Y.; Zhang, X.; Cong, X.Q. Eco friendly fabrication of gold nanoclusters and their induction of cardiomyocyte apoptosis after intratracheal instillation in rats. J. Clust. Sci. 2019. [Google Scholar] [CrossRef]
- Salimi, A.; Rahimi, H.R.; Forootanfar, H.; Jafari, E.; Ameri, A.; Shakibaie, M. Toxicity of microwave-assisted biosynthesized zinc nanoparticles in mice: A preliminary study. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1846–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maksoud, E.M.A.; Lebda, M.A.; Hashem, A.E.; Taha, N.M.; Kamel, M.A. Ginkgo biloba mitigates silver nanoparticles-induced hepatotoxicity in Wistar rats via improvement of mitochondrial biogenesis and antioxidant status. Environ. Sci. Pollut. Res. 2019, 26, 25844–25854. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R.; Szymanski, M.; Przekop, R. The study of toxicity effects of biosynthesized silver nanoparticles using Veronica officinalis extract. Int. J. Environ. Sci. Technol. 2019, 16, 8517–8526. [Google Scholar] [CrossRef] [Green Version]
- Jafarirad, S.; Ardehjani, P.H.; Movafeghi, A. Are the green synthesized nanoparticles safe for environment? A case study of aquatic plant Azolla filiculoides as an indicator exposed to magnetite nanoparticles fabricated using microwave hydrothermal treatment and plant extract. J. Environ. Sci. Health. A 2019, 54, 516–527. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.C.; Jeon, G.E.; Kim, C.S.; Seo, J.H. Effect of the size and shape of silver nanoparticles on bacterial growth and metabolism by monitoring optical density and fluorescence intensity. Biotechnol. Bioproc. E 2017, 22, 210–217. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio natriegens. PLoS ONE 2019, 14, 0222322. [Google Scholar] [CrossRef] [Green Version]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Diţu, L.M.; Ungureanu, C.; Sutan, A.N.; Drăghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1915–1932. [Google Scholar] [CrossRef]
- Lin, Q.; Hong, X.; Zhang, D.; Jin, H. Biosynthesis of size-controlled gold nanoparticles using M. lucida leaf extract and their penetration studies on human skin for plastic surgery applications. J. Photochem. Photobiol. B Biol. 2019, 199, 111591. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H. Topical Silver Nanoparticles for Microbial Activity. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03752424 (accessed on 28 December 2019).
- Aghajanzadeh, H. Antimicrobial Effects of Nanoparticles in Complete Prostheses. 2019. Available online: https://en.irct.ir/trial/38575 (accessed on 28 December 2019).
- Joshi, I. Use of Silver Nanoparticles for the Treatment of Pyorrhea. 2019. Available online: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=33021 (accessed on 28 December 2019).
- Boroumand, Z.; Golmakani, N.; Mazloum, S.R.; Dadgar, S.; Golmohamadzadeh, S. The Effect of Spray Silver Nanoparticles (Nivasha) on Intensity of Cesarean Wound Pain; A Randomized Clinical Trial. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01697748 (accessed on 28 December 2019).
- Antonelli, M. Comparison of Central Venous Catheters with Silver Nanoparticles versus Conventional Catheters (NanoAgCVC). 2011. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00337714 (accessed on 28 December 2019).



| Extract Used | NPs Characteristics | Microbial Lines | Antimicrobial Effect | Ref. |
|---|---|---|---|---|
| Tecoma stans (L.) Juss. ex Kunth flowers aq. extract | Spherical, 50–60 nm | Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria. | IZ = 24/16 mm | [14] |
| Bidens Pilosa L. 1753 leaves, stem and root aq. extract | Spherical, 7.85–26.11 nm | Gram-negative (Pseudomonas aeruginosa, Klebsiella pneumonia, Escherichia coli), Gram-positive bacteria (Enterococcus faecalis, Staphylococcus aureus), fungi (Candida albicans/C. krusei/C. parapsilosis). | IE = 52%–56.6%/45.9%–56.1%/48.2%–58.4%/42.2%–46.4%/39.6%/47.4%/67.1%–88.77%/80%–88.3%/88.2%–94.7% at 200 mg/L, | [15] |
| Juniperus procera Hochst. ex Endl. leaves ac. extract | Spherical, cubic, 30–90 nm | Gram-positive (Bacillus subtilis, Micrococcus luteus), Gram-negative bacteria (Proteus mirabilis, Klebsiella pneumoniae), fungi (Candida albicans). | IZ = 28/28/29/18/24 mm | [16] |
| Berberis vulgaris L. leaves and root aq. extract | Spherical, 30–70 nm | Gram-positive (S. aureus), Gram-negative bacteria (E. coli). | MIC = 400/100 mg/L | [17] |
| Trigonella foenum-graecum L. aq. extract | Spherical, 33.93 nm (average diameter) | E. coli, Klebsiella pneumoniae, S. aureus, Salmonella typhi, Pseudomonas aeruginosa, Aspergillus flavus, C. albicans, Trichophyton rubrum, Penicilium notatum, Trichoderma viridiae. | MIC = 125/250/62.5/500/500/250/500/250/500/250 mg/L | [18] |
| Salvia hispanica L. seeds aq. extract | Spherical, 1–23 nm | E. coli, S. aureus | IZ = 18.5/15.3 mm at 7.5 μg | [19] |
| Selaginella bryopteris leaves meth.: aq. (1:1) extract | Spherical, 5–10 nm | E. coli, S. aureus, A. niger | MIC = 25/25/100 mg/L | [20] |
| Paulownia tomentosa (Thunb.) Steud. leaves eth., isoprop., aq. Soxhlet extract | Mainly spherical, 10–45 nm | P. aeruginosa, S. aureus | Visible inhibition zone (not quantified) | [21] |
| Persea americana Mill. seed aq. extract | Spherical, oblongated, 50 nm | E. coli | IZ = 1.5 mm at 334.11 mg/L | [22] |
| Bergenia ciliate (Haw.) Sternb. 1831, Bergenia stracheyi (Hook.f. and Thorns.) 1868, Rumex dentatus L., Rumex hastatus D.Don | Spherical, 25–73 nm | S. aureus, S. haemolyticus, B. cereus, E. coli, S. typhi, P. aeruginosa | MIC = 0.25–1 (P. aeruginosa, S. typhi); 0.25–0.75 (S. aureus); 0.25–0.5 (E. coli), 0.75 (B. cereus), 4 mg/mL (S. haemolyticus) | [23] |
| Tropaeolum majus L. leaves aq. Soxhlet extract | Spherical, 35–55 nm | S. aureus, E. faecalis, E. coli, S. typhi, P. aeruginosa, A. niger, C. albicans, P. notatum, Trichoderma viridiae, Mucor sp. | Inhibition of the tested strains, no quantification provided | [24] |
| Coriandrum sativum L. leaves aq. extract | Spherical, 11.9 nm | Pasteurella multocida, Enterobacter aerogenes, S. aureus, B. subtilis | IZ = 10/11/12/14 mm at 200 μL | [25] |
| Allium sativum L. aq. extract | Spherical, 10–50 nm | S. aureus, P. aeruginosa | IZ = 17.4/19.2 mm at 100 mg/L | [26] |
| Oldenlandia umbellata L. leaves aq. extract | Spherical, 22.7 nm | Streptococcus mutans, S. aureus, E. coli, P. aeruginosa | MIC = 1.5/2.5/0.5/1.5 mg/L | [27] |
| Juniperus chinensis L. leaves 80% meth. extract | Heterogenous morphology, 18–25 nm | E. coli, P. aeruginosa, S. aureus, B. subtilis | MIC = 15/14/18/17 mg/L | [28] |
| Artocarpus integer Spreng. leaves aq. extract | Spherical, 5.76–19 nm | S. aureus, B. cereus, E. coli, Salmonella entertica | IZ = 14/17/15/16 mm at 25 μg | [29] |
| Artemisia absinthium L. aq. extract | Pseudospherical, 2–80 nm | C. albicans, C. parapsilosis, C. krusei | MIC = 0.325 mg/L at a 6:4 nitrate/extract ratio | [30] |
| Clinacanthus nutans (Burm.f.) Lindau leaves meth. extract | Spherical, 77.8–85.3 nm | B. subtilis, E. faecalis, S. aureus, E. coli, P. aeruginosa, Proteus vulgaris | IZ = 11.5/8.33/8.67/8.5/9/8.8 mm at 10 μL | [31] |
| Murraya koenigii L. leaves aq. extract | Spheroidal, 5–20 nm | S. aureus, E. coli | MIC = 32/16–64 mg/L | [32] |
| Myrtus communis L. leaves aq. extract | Spherical, 5–30 nm | S. aureus, E. coli | MIC = 12.5/25 mg/L | [33] |
| Fagus sylvatica L. bark aq. extract | Spherical, polygonal, triangular, 32–62 nm (pH-dependent) | S. aureus, E. coli, Klebsiella pneumoniae, P. aeruginosa | MIC = 0.09–0.34/0.19–0.54/0.99–2.74/0.15–0.41 mg/mL (dependent on metal source) | [34] |
| Scorzonera calyculata Boiss aerial part eth.: aq. extract | Spherical, 25.28 nm | S. aureus, Listeria monocytogenes, B. subtilis, K. pneumoniae, P. aeruginosa, S. pyogenes | MIC = 125/62.5/125/31.25/62.5/250 mg/L | [35] |
| Derris trifoliata Lour seeds aq. extract | Spherical, 16,92 nm | K. pneumonia, S. aureus, E. coli, P. aeruginosa | IZ = 20/36/19.5/ absent at 0.03 mg | [36] |
| Azadirachta indica A.Juss., 1830 leaves aq. extract | Spherical, 29 nm | Penicillium sp., Fusarium sp., Aspergillus sp. Ralstonia solanacearum | 92%/89%/69% inhibition after 6 days, respectively, MIC = 200 mg/L | [37] |
| Impatiens balsamina L., Lantana camara L. leaves aq. extracts | Spherical, 12–20/3.2–12 nm | S. aureus, E. coli | IZ = 11.03–13.8/13.9–15.8 mm (S. aureus), 8.9–10.2/15.4–17.7 (E. coli) | [38] |
| Rosa santana petals aq. extract | Spherical, 6.52–25.24 nm | S. aureus, E. coli | IZ = 11.73/10.20 mm | [39] |
| Reinwardtia indica Dumort. leaves eth. Soxhlet extract | Spherical, 3–15 nm | S. aureus, E. coli, P. aeruginosa, C. albicans | IZ = 14.2/13.6/15.9/14.1 mm | [40] |
| Morus alba L. fruits aq. extract | Spherical, 80–150 nm | E. coli, L. monocytogenes | IZ = 24.87/26.93 at 5% | [41] |
| Uncaria gambir Roxb. leaves aq. extract | Spherical, 6–41 nm | S. aureus, E. coli | IZ = 16/14 mm | [42] |
| Olea europaea L. leaves aq. extract | Spherical, 10–22 nm | Coliforms | Absence of colonies at 50 mg/L | [43] |
| Corymbia citriodora (Hook.) K.D. Hill and L.A.S. Johnson leaves eth. extract | Spherical, 17.51 nm | Acinetobacter baumannii, E. coli, P. aeruginosa, K. pneumoniae, E. faecalis, S. aureus, C. albicans | MIC90 = 0.04/0.04/0.04/0.04/0.04/0.09/0.02 mg/L | [44] |
| Stachys lavandulifolia flower aq. extract | Spherical 20–40 nm | P. mirabilis, Shigella flexneri, L. monocytogenes, K. pneumonia, P. aeruginosa, E. coli, E. faecalis, B. subtilis, Streptococcus pyogenes, Staphylococcus saprophyticus, S. epidermidis, S. aureus, S. typhimurium, Streptococcus pneumonia | IZ = 39.8–49.2 mm at 64 mg/mL | [45] |
| Salvia miltiorrhiza Bunge leaves aq. extract | Spherical, oval, hexagonal and Triangular, 12–80 nm | S. typhi, S. flexneri, S. pyogenes, P. aeruginosa | IZ = 10.2/10.5/10.8/9.24 at 60 μg | [46] |
| Allium cepa L. peel aq. extract | Spherical, 8–50 nm | Bacillus sp., S. aureus, Corynebacterium sp., E. coli, Salmonella sp., Vibrio cholerae | IZ = 17/19/17/19.3/17.7/18 mm at 100 mg/L | [47] |
| NPs | Extract Used | NPs Characteristics | Microbial Lines | Antimicrobial Effect | Ref. |
|---|---|---|---|---|---|
| Au NPs | Mix of Olea europaea L. fruit and Acacia nilotica (L.) Wild. ex Delile husk aq. extracts | Spherical, with irregular forms, 44.96 nm | Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis | >4 mm inhibition zones, superior for Gram-negative bacteria | [50] |
| Au NPs | Tribulus terrestris L. fruit aq. extract | Spherical, few triangular, 7 nm (1 mM metal salt precursor)/55 nm (2 mM) | Helicobacter pylori | IZ = 10.2–12.1 mm, MIC = 16.75–21.50 mg/L | [51] |
| Au NPs | Gundelia tournefortii L. leaves aq. extract | Spherical, 40–45 nm | C. albicans, C. glabrata, C. krusei, C. guilliermondii, P. aeruginosa, E. coli, B. subtilis, S. aureus, Salmonella typhimurium, Streptococcus pneumonia | IZ = 33–38 mm at 64 mg/mL (against Candida sp.), MIC/MBC = 2–4 mg/mL | [52] |
| Au NPs | Anacardium occidentale L. leaves aq. extract | Spherical, 10–60 nm | E. coli, B. subtilis | IZ = 24/10 mm at 40 μL | [53] |
| Au NPs | Halymenia dilatate Zanardini aq. extract | Triangular, spherical, 16 nm | Aeromonas hydrophila | IZ = 21 mm at 100 mg/L | [54] |
| Cu NPs | Allium saralicum R.M. Fritsch leaves aq. extract | Spherical, 45–50 nm | C. albicans, C. glabrata, C. krusei, C. guilliermondii, P. aeruginosa, E. coli, B. subtilis, S. aureus, S. typhimurium, S. pneumonia | MFC = 2–8 mg/mL, MBC = 4–8 mg/mL (Gram-negative), 2–8 mg/mL (Gram-positive) | [55] |
| Fe NPs | Uvaria chamae P. Beauv. leaves aq. extract | Irregular shapes, 29.5–51.1 nm | E. coli, P. aeruginosa, B. subtilis, S. aureus, C. albicans, A. niger | IZ = 11/11/14/15/17/16 mm at 2 mg/mL, MIC = 0.5 mg/mL | [56] |
| Ti NPs | Allium eriophyllum Boiss leaves aq. extract | Spherical, 22 nm | C. guilliermondii, C. krusei, C. albicans, C. glabrata, P. aeruginosa, S. typhimurium, E. coli, S. aureus, S. pneumoniae, B. subtilis | MFC = 8–16 mg/mL, MBC = 4 mg/mL | [57] |
| Se NPs | Emblica officinalis Gaertn. fruits aq. extract | Spherical, 15–40 nm | E. coli, Listeria monocytogenes, S. aureus, Enterococcus faecalis, A. brasiliensis, A. flavus A. oryzae, A. ochraceus, Fusarium anthophilum, Rhizopus stolonifer | MBC = 33.17–97.5 mg/L, MFC = 10.67–38.17 mg/L | [58] |
| ZnO NPs | Tecoma castanifolia (D.Don) Melch. leaves aq. extract | Spherical, 70–75 nm | E. coli, P. aeruginosa, S. aureus, B. subtilis | IZ = 17/15/17/15 mm at 100 μg | [59] |
| ZnO NPs | Bambusa vulgaris Schrad. ex J.C.Wendl., Artabotrys hexapetalus (L. f.) Bhandari leaves aq. extracts | Spherical/spherical and rod-shaped, 15–20/20–30 nm | Streptococcus and Serratia strains | IZ = 6/5 (Streptococcus), 15/13 (Serratia) mm | [60] |
| ZnO NPs | Pandanus odorifer (Forssk.) Kuntze leaves aq. extract | Spherical, 90 nm | B. subtilis E. coli | IZ = 26/24 mm at 50 μg/well | [61] |
| ZnO NPs | Cinnamomum tamala (Buch.-Ham.) T. Nees and C. H. Eberm. leaves aq. extract | Spherical, hexagonal, 26.57 nm | S. aureus | 20% inhibition at 100 mg/L | [62] |
| ZnO NPs | Rhamnus virgate Roxb. leaves aq. extract | Hexagonal, triangular, 20–30 nm | E. coli, S. aureus, B. subtilis, K. pneumonia, P. aeruginosa, C. albicans, Mucor racemosus, A. niger, Fusarium solani, A. flavus | MIC = 7.8–125 mg/L, best results for S. aureus and B. subtilis | [63] |
| ZnO NPs | Euphorbia heterophylla L. leaves aq. Soxhlet extract | Hexagonal, 40 nm | S. aureus, E. coli, Pseudomonas desmolyticum, Klebsiella aerogenes | IZ = 10.83/8.43/8.92/6.5 at 1000 mg/L | [64] |
| ZnO NPs | Mentha pulegium L. leaves aq. extract | Semi-spherical, 38–49 nm | S. aureus, E. coli | IZ = 22.7/19.3 at 200 mg/L | [65] |
| ZnO NPs | Allium cepa L. leaves aq. extract | Hexagonal, cubic, 50 nm | Bacillus sp., E. coli, S. aureus, Vibrio cholerea, Corynebacterium sp., Salmonella sp. | IZ = 20.33/20.33/20/18.33/16/17 mm at 100 mg/L | [66] |
| ZnO NPs | Laurus nobilis L. leaves aq. extract | Spherical, hexagonal, 20–30 nm | E. coli | MIC = 1200 mg/L | [67] |
| Fe2O3 NPs | Rhamnus virgate Roxb. leaves aq. extract | Spherical, 20 nm | S. aureus, B. subtilis, P. aeruginosa, Klebsiella pneumoniae, E. coli, M. racemosus, A. flavus, A. niger, C. albicans, F. solani | MIC = 31.25–125 mg/L | [68] |
| Fe3O4 NPs | Artemisia haussknechtii Boiss. leaves aq. extract | Dendrimer shaped, with branches, 1–150 nm | E. coli, S. aureus, S. marcescens | IZ = 12.16–13.16 at 0.1 M metallic salt precursor, MIC = 50/12.5/50 mg/L | [69] |
| MgO NPs | Sargassum wightii Greville ex J. Agardh, 1848 aq. extract | Flower shaped, 68.02 nm | S. aureus, P. aeruginosa | IZ = 9/8 mm at 30 mg/L, MIC = 256 mg/L, MBC = 256/1024 mg/L | [70] |
| MgO NPs | Rosmarinus officinalis L. flowers aq. extract | Flower shaped, 8.8 nm | Xanthomonas oryzae pv. oryzae | IZ = 5.1 cm at 16 mg/L | [71] |
| NiO NPs | Abelmoschus esculentus (L.) Moench leaves aq. extract | Spherical, 18.6 nm | S. aureus, E. coli, P. aeruginosa | IZ = 10 mm (S. aureus) | [72] |
| CoO NPs | Geranium wallichianum Oliv. leaves aq. extract | 21 nm | B. subtilis, S. aureus, P. aeruginosa, E. coli, K. pneumonia, M. racemosus, C. albicans, A. niger, A. flavus, F. solanai | MIC = 21.875/87.5/175/43.75/175/21.875/43.75/21.875/175/21.875 mg/L | [73] |
| Ag/TiO2 NPs | Acacia nilotica (L.) Wild. ex Delile leaves aq. extract | Spherical, 17 nm | S. aureus, E. coli, P. aeruginosa, C. albicans | IZ = 64/64/128/64 mg/L | [74] |
| Au/Ag NPs | Annona squamosa L. aq. extract | Multiple morphologies (spherical, triangular, hexagonal, rod-shaped, etc.) 30–50 nm | B. subtilis, S. aureus, E. coli, S. typhi | IZ = 14.66/13.66/11/9.33 mm | [75] |
| Au/Ag NPs | Piper betle L. leaves aq. extract | Clusters, spherical | B. subtilis, K. planticola. | IZ = 14/13 mm at 50 μL | [76] |
| Au, Ag, Au/Ag NPs | Persea americana Mill. fruit peel aq. extract | Spherical, rod-shaped aggregates, 16–70/18–80/44–55 nm | E. coli, B. subtilis, K. pneumoniae, L. monocytogenes, P. vulgaris, P. aeruginosa, S. aureus, S. pyogenes, A. niger, A. fumigatus, F. solani, A. flavus, C. albicans | IE = 36%–76%/52%–94%/53%–85% at 80 mg/L | [77] |
| Au, ZnO and Au/ZnO core-shell NPs | Hibiscus sabdariffa L. leaves aq. extract | Spherical, 20–50 nm | S. aureus | Complete inhibition at 500/750 mg/L after 3 h | [78] |
| Fe3O4, Cu, Fe3O4/Cu NPs | Carum carvi L. seeds aq. extract | Spherical, 25/37/62 nm | S. aureus, B. subtilis, E. coli | MIC = 0.01/0.05/0.02 (Cu)/0.01/0.04/0.03 (composite) | [79] |
| NPs | Extract Used | NPs Characteristics | Cell Lines | Main Findings | Ref. |
|---|---|---|---|---|---|
| Ag NPs | Bidens pilosa L. 1753 leaves, stem and root aq. extract | Spherical, 7.85–26.11 nm | A549 | MTS assay—CD = 55.6%/44.9%/43.5% | [15] |
| Ag NPs | Juniperus procera Hochst. ex Endl. leaves ac., eth. extracts | Spherical, cubic, 30–90 nm | Cow RBC | Lysis effect: 1.75%/100% | [16] |
| Ag NPs | Trigonella foenum-graecum L. aq. extract | Spherical, 33.93 nm | MCF7 | MTT assay—EC50 = 6.25 mg/L | [18] |
| Ag NPs | Tropaeolum majus L. leaves aq. Soxhlet extract | Spherical, 35–55 nm | MCF7 | MTT assay—EC50 = 2.49 mg/L | [24] |
| Ag NPs | Allium sativum L. aq. extract | Spherical, 10–50 nm | MCF7 | MTT assay—EC50 = 23 mg/L | [26] |
| Ag NPs | Artocarpus integer Spreng. leaves aq. extract | Spherical, 5.76–19 nm | MCF7, MG-63 | MTT assay—EC50 = 90/70 mg/L after 24 h | [29] |
| Ag NPs | Scorzonera calyculata Boiss aerial part eth.:aq. extract | Spherical, 25.28 nm | A549 | MTT assay—EC50 = 12.5 mg/L | [35] |
| Ag NPs | Derris trifoliata Lour seeds aq. extract | Spherical, 16,92 nm | A549 | MTT assay—EC50 = 86.23 mg/L after 24 h | [36] |
| Ag NPs | Reinwardtia indica Dumort. leaves eth. Soxhlet extract | Spherical, 3–15 nm | SiHa | MTT assay—CV = approx. 10% after 24 h at 500 mg/L | [40] |
| Ag NPs | Olea europaea L. leaves aq. extract | Spherical, 10–22 nm | MCF7, HeLa | CV = 48%/38% after 96 h, at 50 mg/L | [43] |
| Ag NPs | Salvia miltiorrhiza Bunge leaves aq. extract | Spherical, oval, hexagonal, triangular, 12–80 nm | LNCaP | MTT assay—CV = approx. 38% after 24 h at 100 mg/L | [46] |
| Ag NPs | Allium cepa L. peel aq. extract | Spherical, 8–50 nm | A549 | MTT assay—EC50 = 113.25 mg/L at 24 h | [47] |
| Ag NPs | Leucas aspera (Willd.) Link leaves aq. extract | Spherical, 50 nm | HeLa | LDH assay—Ctx = 58% after 24 h at 150 mg/L | [82] |
| Ag NPs | Ceiba pentandra L. bark eth. extract | Spherical, 5–50 nm | HCT-116 | MTT assay—EC50 = 60 mg/L | [83] |
| Ag NPs | Jasminum sambac L. (Ait) leaves eth. Extracts—two cultivars | Spherical, 8.83/11.24 nm | MCF7, 5637 | EC50 = 6.32/17.32 (MCF7) 5.54/27.89 (5637) mg/L | [84] |
| Ag NPs | Datura inoxia Mill. flowers aq. extract | Polygonal, 15–73 nm | MCF7 | MTT assay—EC50 = 20 mg/L after 24 h | [85] |
| Ag NPs | Phoenix dactylifera Chabaud seed eth. extract | Spherical, 17–19 nm | MCF7 | MTT assay—EC50 = 188 mg/L | [86] |
| Ag NPs | Nigella sativa L. seeds aq. extract | Spherical, 100–150 nm | MCF7 | MTT assay—EC50 = 10 g/L for 24 h | [87] |
| Ag NPs | Piper longum L. leaves aq. extract | Spherical, 28.8 nm | HeLa | MTT assay—EC50 = 5.27 mg/L after 24 h | [88] |
| Ag NPs | Manilkara zapota (L.) P. Royen leaves aq. extract | Spherical, 24 nm | HCT-116, HeLa, A549 | MTT assay—EC50 = 8/16/29 mg/L | [89] |
| Ag NPs | Dysosma pleiantha (Hance) Woodson rhizomes aq. extract | Spherical, 76 nm | MDA-MB-231, MDA-MB-453, AGS | MTT assay—EC50 = 33.521/36.25/7.14 mM/L | [90] |
| Ag NPs | Elaeodendron croceum (Thunb.) DC. stem bark aq. extract | Spherical, 12.62–41.44 nm | MDA-MB-231 | WST-1 method, EC50 = 138.8 mg/L | [91] |
| Ag NPs | Rauvolfia tetraphylla L. leaves aq. extract | Spherical, 40 nm | Allium cepa assay; MCF7, A549 | Antimitotic activity, chromosomal aberrations; MTT assay—EC50 = 134.67/118.5 mg/L | [92] |
| Ag, Au NPs | Aconitum toxicum Reichenb. leaves eth., meth. extracts | Spherical, 12.22/13.45 (Au), 21.96/22.08 (Ag) nm | Allium cepa assay | Antimitotic activity, chromosomal aberrations | [93] |
| Au NPs | Halymenia dilatate Zanardini aq. extract | Triangular, spherical, 16 nm | HT-29 | MTT assay—EC50 = 22.62 mg/L | [54] |
| Au NPs | Mix of Olea europaea L. fruit and Acacia nilotica (L.) Wild. ex Delile husk aq. extracts | Spherical, with irregular forms, 44.96 nm | MCF7, TCT-116, HCepG-2 | MTT assay—EC50 = 45.5/37.2/40.6 μL | [50] |
| Au NPs | Anacardium occidentale L. leaves aq. extract | Spherical, 10–60 nm | MCF7 | MTT assay—EC50 = 6 mg/L | [53] |
| Au NPs | Tribulus terrestris L. fruit aq. extract | Spherical, few triangular, 7 nm (1 mM metal salt precursor)/55 nm (2 mM) | AGS | Annexin V/Propidium Iodide staining assay CV > 70% at 24 h, for both types of NPs at 200 mg/L | [51] |
| Au NPs | Lonicera japonica L. flowers aq. extract | Spherical, triangular, hexagonal, 10–40 nm | HeLa | WST-1 method, CV = approx. 50% at 400 mg/L | [94] |
| Au NPs | Marsdenia tenacissima (Roxb.) Moon leaves aq. extract | Spherical, oval-shaped, 40–50 nm | A549 | MTT assay—EC50 = 15 mg/L | [95] |
| Au NPs | Camellia sinensis (L.) Kuntze leaves aq. extract | Spheres, stars, 8.7/99 nm | AGS, HeLa, HepG2, HT-29 | Cytotoxic towards all lines, MTT assay—EC50 = 127.1/81.8 μM (HepG2) | [96] |
| Au NPs | Citrus macroptera Mont. fruit juice | Pseudospherical trigonal, rod-shaped, 20 nm | A549, MDA-MB 468, HepG2 | MTT assay, EC50 = 143/157.9/70.2 μg/L | [97] |
| Au NPs | Panax notoginseng (Burkill) F. H. Chen ex C. Y. Wu and K. M. Feng leaves aq. extract | Hexagonal, spherical, oval, triangular, 12–80 nm | PANC-1 | MTT assay—CV = approx. 25% after 48 h at 30 mg/L | [98] |
| Au NPs | Alternanthera sessilis (L.) R.Br. ex DC. leaves aq. extract | Spherical, 30–50 nm | HeLa | MTT assay—EC50 = 10 mg/L after 24 h | [99] |
| Au NPs | Eleutherococcus senticosus (Rupr. and Maxim.) Maxim leaves and stems aq. extract | Spherical, 20 nm | B16 | MTT assay—EC50 = 10 mg/L after 24 h | [100] |
| Au NPs | Ocimum tenuiflorum leaves aq. extract | Spherical, 2–10 nm | HeLa, MCF7, A549, H1299 | MTT assay—EC50 = 200/~180/~220/~350 mg/L after 24 h | [101] |
| Au NPs | Rabdosia rubescens L. leaves aq. extract | Spherical, 130 nm | A549 | MTT assay—EC50 = 50 mg/L after 24 h | [102] |
| Au NPs | Dunaliella salina (Dunal) Teodoresco aq. extract | Spherical, triangular, hexagonal, 5–45 nm | MCF7 | MTT assay—CV = 20% after 48 h at 200 mg/L | [103] |
| NPs | Extract Used | NPs Characteristics | Cell Lines | Main Findings | Ref. |
|---|---|---|---|---|---|
| ZnO NPs | Tecoma castanifolia (D.Don) Melch. leaves aq. extract | Spherical, 70–75 nm | A549 | MTT assay—EC50 = 65 mg/L | [59] |
| ZnO NPs | Pandanus odorifer (Forssk.) Kuntze leaves aq. extract | Spherical, 90 nm | MCF7, HepG2, A549 | MTT assay—CV < 65% after 24 h, at 100 mg/L | [61] |
| ZnO NPs | Rhamnus virgate Roxb. leaves aq. extract | Hexagonal, triangular, 20–30 nm | HepG2 | MTT assay—EC50 = 19.67 mg/L | [63] |
| ZnO NPs | Euphorbia heterophylla L. leaves aq. Soxhlet extract | Hexagonal, 40 nm | A549, HepG2 | MTT assay—EC50 = 383.05/329.67 mg/mL | [64] |
| ZnO NPs | Allium cepa L. leaves aq. extract | Hexagonal, cubic, 50 nm | A549 | MTT assay—EC50 = 51.25 mg/L | [66] |
| ZnO NPs | Hyssops officinalis L. aq. extract | Pseudo-spherical, 20–40 nm | MDA-MB-231, MCF7 | MTT assay—CV = 7/4% after 72 h at 500/100 mg/L | [104] |
| ZnO NPs | Rheum turkestanicum Janisch rhizome aq. extract | Spherical, 32.9 nm | WEHI 164 | MTT assay—EC50 = 212.5 mg/L | [105] |
| ZnO NPs | Scutellaria baicalensis Georgi roots aq. extract | Spherical, 33.14–99.03 nm | HeLa | XTT assay—CV = 59.03% at 1000 mg/L | [106] |
| ZnO NPs | Gracilaria edulis (S.G.Gmelin) P.C.Silva aq. extract | Rod-shaped, 1.39 nm | SiHa | MTT assay—EC50 = 35 mg/L | [107] |
| ZnO NPs | Annona squamosa L. leaves aq. extract | Hexagonal, 20–50 nm | HeLa | MTT assay—EC50 = 50 mg/L | [108] |
| ZnO NPs | Artocarpus heterophyllus Lam. leaves aq. extract | Spherical, 12–24 nm | HCT-116 | MTT assay—EC50 = 20 mg/L | [109] |
| Fe2O3 NPs | Rhamnus virgate Roxb. leaves aq. extract | Spherical, 20 nm | HepG2 | MTT assay—EC50 = 13.47 mg/L | [68] |
| Fe3O4 NPs | Cydonia oblonga Miller seeds aq. Extract | Spherical, <50 nm | A549 | MTT assay—CV approx. 40%, at 100 mg/L | [110] |
| Fe2O3, PbO NPs | Papaver somniferum L. pods aq. extract | Elliptical, spherical, 38 nm/Irregular, 23 nm | HepG2 | SRB method—CV = 20.88%/38.49% after 24 h at 200 mg/L | [111] |
| Fe NPs | Camellia sinensis (L.) Kuntze leaves aq. extract | Spherical, 31.84 nm | SW1353 | MTT assay—CV = 62% at 150 mg/L | [112] |
| CeO2 NPs | Origanum majorana L. leaves aq. extract | Spherical, 20 nm | MDA-MB-231 | MTT assay—CV = 41.47% after 48 h at 125 mg/L | [113] |
| CeO2 NPs | Ceratonia siliqua L., 1753 leaves aq. extract | Spherical, 22 nm | MCF7 | MTT assay—CV = 38.67% after 72 h at 1000 mg/L | [114] |
| CeO2 NPs | Salvadora persica L. bark aq. extract | Spherical, 10–15 nm | HT-29 | MTT assay—CV = 80% after 24 h at 800 mg/L | [115] |
| CoO NPs | Geranium wallichianum Oliv. leaves aq. extract | 21 nm | HepG2 | MTT assay—EC50 = 31.4 mg/L | [73] |
| MgO NPs | Sargassum wightii Greville ex J.Agardh, 1848 aq. extract | Flower shaped, 68.02 nm | A549 | MTT assay—EC50 = 37.5 mg/L | [70] |
| NiO NPs | Abelmoschus esculentus(L.) Moench leaves aq. extract | Spherical, 18.6 nm | Neuro2a | MTT assay—CV approx. 58% at 500 mg/L | [72] |
| Se NPs | Spermacoce hispida L. leaves aq. extract | Spherical, 50 nm | HepG2 | MTT assay—CV = 50% at 30 mg/L | [116] |
| ZnS NPs | Stevia rebaudiana Bertoni leaves aq. Extract | Spherical, 8.35 nm | MCF7 | MTT assay—EC50 = 400 mg/L | [117] |
| CuO, ZnO, CuO/ZnO NPs | Alchornea cordifolia Müll.Arg. leaves aq. extract | Spherical, star-like (for the composite), 16.25/75.22/3.54 nm | HeLa | MTT assay—CV = 63.64/44.05/39.94 after 48 h at 100 mg/L | [118] |
| Ag/TiO2 NPs | Acacia nilotica (L.) Wild. ex Delile leaves aq. extract | Spherical, 17 nm | MCF7 | MTT assay—CV approx. 45% after 24 h at 100 μM | [74] |
| Fe3O4/Au NPs | Juglans regia L. husk aq. extract | Core-shell, 6.08 nm | HT-29 | MTT assay—EC50 = 235 mg/L | [119] |
| NPs | NPs Characteristics | Cell Lines | Main Findings | Ref. |
|---|---|---|---|---|
| Ag NPs | Spherical, 33.93 nm | VERO | MTT assay—EC50 = 12.5 mg/L | [18] |
| Ag NPs | Spherical, 35–55 nm | VERO | MTT assay—EC50 = 5.3 mg/L | [24] |
| Ag NPs | Spherical, 10–50 nm | HEK-293 | MTT assay—EC50 = 23 mg/L | [26] |
| Ag NPs | Spherical, 22.7 nm | WI-38 | CV = 90% at 100 mg/L | [27] |
| Ag NPs | Spherical, 5.76–19 nm | 3T3 | MTT assay—EC50 = 110 mg/L after 24 h | [29] |
| Ag NPs | Spherical 20–40 nm | HUVEC | MTT assay—EC50 = 760 mg/L | [45] |
| Ag NPs | Spherical, 8.83/11.24 nm | HaCaT | EC50 = 490/300 mg/L | [84] |
| Ag NPs | Spherical, 28.8 nm | HEK-293 | MTT assay—EC50 = 1844 mg/L after 24 h | [88] |
| Ag NPs | Spherical, 24 nm | hPBLs | MTT—CV = 70% at 80 mg/L | [89] |
| Au NPs | Spherical, 10–60 nm | PBMC | MTT assay—EC50 = 600 mg/L | [53] |
| Au NPs | Spherical, triangular, hexagonal, 10–40 nm | HEK-293 | WST-1 method, CV > 95% at 500 mg/L | [94] |
| Au NPs | Spherical, 2–10 nm | HEK-293 | MTT assay—CV > 80% at 400 mg/L after 24 h | [101] |
| Au NPs | Spherical, triangular, hexagonal, 5–45 nm | MCF-10A | MTT assay—CV = not affected after 48 h at 200 mg/L | [103] |
| ZnO NPs | Hexagonal, triangular, 20–30 nm | RBC | MTT assay—EC50 > 200 mg/L | [63] |
| ZnO NPs | Hexagonal, 20–50 nm | HEK-293 | MTT assay—CV = 76% at 200 mg/L | [108] |
| ZnO NPs | Spherical, 12–24 nm | VERO | MTT assay—EC50 = 30 mg/L | [109] |
| Fe2O3 NPs | Spherical, 20 nm | RBC | MTT assay—EC50 > 200 mg/L | [68] |
| Fe2O3, PbO NPs | Elliptical, spherical, 38 nm/Irregular, 23 nm | RBC | SRB method—CV = 59%/50.3% after 24 h at 400 mg/mL | [111] |
| Fe NPs | Spherical, 31.84 nm | VERO | MTT assay—CV = 80% at 150 mg/L | [112] |
| Se NPs | Spherical, 15–40 nm | N2a | MTT assay—EC50 = 127.28 mg/L | [58] |
| Se NPs | Spherical, 50 nm | VERO | MTT assay—CV not affected after 48 h at 60 mg/L | [116] |
| Cu NPs | Spherical, 45–50 nm | HUVEC | MTT assay—CV > 85% after 48 h at 1000 mg/L | [54] |
| CeO2 NPs | Spherical, 20 nm | HUVEC | MTT assay—CV = 87.67% after 72 h at 1000 mg/L | [113] |
| CeO2 NPs | Spherical, 23 nm | Lymphocytes | MTT assay—CV = 99.38% at 2.5 mg/L | [114] |
| CoO NPs | 21 nm | Human macrophages and erythrocytes | MTT assay—EC50 > 200 mg/L | [73] |
| MgO NPs | Flower shaped, 68.02 nm | PBMC | MTT assay—CV > 95% after 24 h at 100 mg/L | [70] |
| Au, ZnO and Au/ZnO core-shell NPs | Spherical, 20–50 nm | Mouse fibroblast cells | MTT assay—CV = >80%/>50%/>70% at 250 mg/L | [78] |
| Fe3O4/Au NPs | Core-shell, 6.08 nm | 3T3 | MTT assay—EC50 > 500 mg/L | [119] |
| NPs | Plant Material | NPs Characteristics | Test Organisms | Main Findings | Ref. |
|---|---|---|---|---|---|
| Ag NPs | Selaginella bryopteris leaves meth.: aq. (1:1) extract | Spherical, 5–10 nm | Mice injected with different doses (10–200 μg) of NPs | No hemorrhage and edema observed in experimental mice up to 100 μg | [20] |
| Ag NPs | Allium sativum L. aq. extract | Spherical, 10–50 nm | Ceriodaphnia cornuta G. O. Sars, 1885 (Daphniidae) exposed to 5–250 μg/L for 24 h | No mortality recorded at to 250 μg/L, affection of the swimming behavior at 250 μg/L (erratic swimming, migration to the bottom of the beaker or the water surface). | [26] |
| Ag NPs | Piper longum L. leaves aq. extract | Spherical, 28.8 nm | Mesocyclops thermocyclopoides Harada, 1931 (Cyclopidae) exposed to 250 solution for 72 h | No toxicity recorded | [88] |
| Ag NPs | Elaeodendron croceum (Thunb.) DC. stem bark aq. extract | Spherical, 12.62–41.44 nm | Acute oral toxicity evaluated on Wistar rats administered 500–2000 mg/kg NP doses | LD50 > 2000 mg/kg, no significant difference for mean organ-to-body weight ratio except in the liver and in all hematological parameters except WBC and hematocrit; no significant difference for serum electrolytes. total protein, urea, GGT, AST, ALP, ALT, albumin, bilirubin; changes in creatinine, urea, and cholesterol levels. | [91] |
| Ag NPs | Solanum nigrum L. leaves aq. extract | Spherical, 10–50 nm | Ceriodaphnia cornuta, Paramecium sp., Poecilia reticulata (guppy fish) | C. cornuta: LC50 = 23.5 mg/L, 100% lethality at 50 mg/L after 24 h, abnormal swimming behavior at lower concentrations; Paramecium: LC50 = 15.5 mg/L, 100% lethality at 30 mg/L after 5 min, morphological deformities (blackening, swelling, spindle shape deformity, blackening of cytoplasm) at lower concentrations; fish: LC50 = 38.3/34.5 mg/L after 48/96 h, 100% mortality at 50 mg/L after 96 h, no mortality under 20 mg/L., heart rate decreased with increasing concentration | [120] |
| Au NPs | Halymenia dilatate Zanardini aq. extract | Triangular, spherical, 16 nm | Danio rerio (F. Hamilton, 1822) (zebrafish) embryo exposed to 0–100 mg/L NPs for 96 h | No mortality or morphology variations after 96 h at 100 mg/L | [54] |
| Au NPs | Cleome viscosa L. leaves aq. extract | Spherical, 1–1.5 nm | Wistar male rats treated with 2, 5, 10 mg/kg released into the lungs | Increased amount of Au in serum and heart, LDH and CK-MB activities, cardiovascular injuries | [121] |
| ZnO NPs | Rhamnus virgate Roxb. leaves aq. extract | Hexagonal, triangular, 20–30 nm | Artemia sp. (brine shrimps) exposed to 1–200 mg/L NPs for 24 h | LC50 = 26.34 mg/L | [63] |
| Zn NPs | Lavandula vera DC. leaves aq. extract | Spherical, 30–80 nm | Oral acute and subacute toxicity in male NMRI mice administered NPs by oral gavage for 14 days | LC50 = 5.5 g/kg (non-toxic); low oral toxicity at 1, 2 and 3 g/kg after 14 days; sub-acute effects—changes in the body weight, hematological parameters, no toxicological effects at 1 g/kg | [122] |
| Fe2O3 NPs | Rhamnus virgate Roxb. leaves aq. extract | Spherical, 20 nm | Artemia sp. (brine shrimps) exposed to 1–200 mg/L NPs for 24 h | LC50 = 32.41 mg/L | [68] |
| CoO NPs | Geranium wallichianum Oliv. leaves aq. extract | 21 nm | Artemia sp. (brine shrimps) exposed to 1–200 mg/L NPs for 24 h | LC50 = 18.12 mg/L | [73] |
| CeO2 NPs | Rhus punjabensis J. L. Stewart ex Brandis stem aq. extract | Spherical, 23 nm | Female Sprague-Dawley rats orally administered doses of 200/400 mg/kg body weight for 14 days | No effect on serum biochemistry, except for creatine phosphokinase (significantly reduced) | [114] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fierascu, I.; Fierascu, I.C.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C. Phytosynthesized Metallic Nanoparticles—between Nanomedicine and Toxicology. A Brief Review of 2019′s Findings. Materials 2020, 13, 574. https://doi.org/10.3390/ma13030574
Fierascu I, Fierascu IC, Brazdis RI, Baroi AM, Fistos T, Fierascu RC. Phytosynthesized Metallic Nanoparticles—between Nanomedicine and Toxicology. A Brief Review of 2019′s Findings. Materials. 2020; 13(3):574. https://doi.org/10.3390/ma13030574
Chicago/Turabian StyleFierascu, Irina, Ioana Catalina Fierascu, Roxana Ioana Brazdis, Anda Maria Baroi, Toma Fistos, and Radu Claudiu Fierascu. 2020. "Phytosynthesized Metallic Nanoparticles—between Nanomedicine and Toxicology. A Brief Review of 2019′s Findings" Materials 13, no. 3: 574. https://doi.org/10.3390/ma13030574
APA StyleFierascu, I., Fierascu, I. C., Brazdis, R. I., Baroi, A. M., Fistos, T., & Fierascu, R. C. (2020). Phytosynthesized Metallic Nanoparticles—between Nanomedicine and Toxicology. A Brief Review of 2019′s Findings. Materials, 13(3), 574. https://doi.org/10.3390/ma13030574